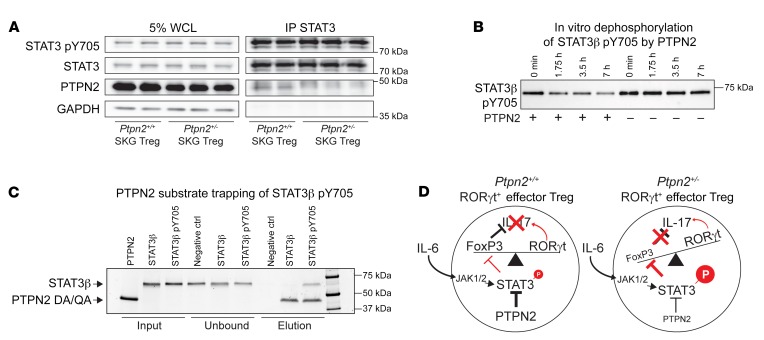
Figure 13. PTPN2 directly interacts with and dephosphorylates STAT3.
(A) Immunoprecipitation of STAT3 in in vitro–expanded Tregs (expanded with IL-2 and anti-CD3/CD28–coated beads) after stimulation with IL-6 (50 ng/ml) for 20 minutes. All samples shown were separated on the same gel. WCL, whole cell lysate. (B) Dephosphorylation of STAT3β pY705 after incubation with (+) or without (–) recombinant PTPN2. Samples were taken at 0, 1 hour 45 minutes, 3 hours 30 minutes, and 7 hours for analysis. All samples shown were separated on the same gel. (C) Substrate trapping of STAT3β pY705 by the PTPN2 mutant (D182A, Q260A). Unphosphorylated STAT3β was used as a negative control and does not bind PTPN2, as shown. Proteins were analyzed by SDS-PAGE and run on the same gel. (D) Schematic of proposed mechanism by which partial loss of function in PTPN2 in Tregs promotes STAT3-mediated loss of FoxP3 and generation of IL-17A–producing exTregs. Representative experiments out of 2 (A) and 3 (B and C) independent replicates are shown. See complete unedited blots in the supplemental material.