Introduction
Healthcare disparities in quality represent one of the greatest challenges in achieving uniformly high-quality care (1). Research reporting disparities in surgical outcomes are abundant (2–6). The cornerstone of delivering high quality healthcare is ensuring optimal access for all patients. A relative lack of access to surgical services may be a contributing factor to disparities in surgical outcomes.
Access is “the timely use of personal health services to achieve the best possible outcomes” (7). Utilization of services, the process of entering and staying in the system, and the actual quality of care received are all involved. Disparities in access arise when the system disproportionately under-performs for a specific group of patients relative to the historically advantaged population (8, 9). Surgery, because of its time sensitive, often high acuity nature, is greatly dependent on access.
In complex surgical systems, there is no established, methodical way of conceptualizing or measuring disparities in surgical access. Efforts to reduce disparities in surgical access require metrics (standardized measurable indicators), which provide the foundation for focused and tailored interventions, which can be applied broadly. The aims of this systematic review were to identify measures of disparities in surgical access in the US (US), produce a conceptual model for operationalizing them, and build an evidence map of this literature.
This study was a part of the American College of Surgeons’ collaborative effort towards reducing disparities in surgical care - MEASUR (Metrics for Equitable Access and care in SURgery). The overarching mission is to ensure optimal access and equitable healthcare for all surgical patients in every setting across the entire surgical continuum of care. Through research and expert consensus, the MEASUR project, will identify and develop measures that capture disparities in surgical care.
Methods
Protocol and registration
This review was prospectively registered in the Prospero International Prospective Register of Systematic Reviews (2018 ID: CRD42018091926). The objective was to identify quantitative measures of surgical access disparities in the US and structure these measures into a novel conceptual model.
We defined a measure as a quantitative primary or secondary study outcome that was assessed across disparity domains (race/ethnicity, socioeconomic status, insurance status, education, geographic location, and other).
Search criteria
Surgical access disparity measures are ill-defined, therefore a broad search strategy was utilized. An existing literature search strategy was employed using the following terms: healthcare disparities, health status disparities and surgery (3). A PubMed search of publications published between January 2008 and March 2018 was conducted. The date last searched was March 16th, 2018. Reference mining was utilized to identify additional publications.
Eligibility criteria
Included studies incorporated quantitative measures of disparities in surgical access. The definition for surgical access was extrapolated from the NASEM healthcare access definition (7); the timely use of surgical services to achieve the best possible outcomes including the utilization of surgical services, the process of entering and staying in the surgical system, and the quality of care received. The specific measurements of surgical access utilized and the disparity domain (race/ethnicity, socioeconomic status, insurance status, education, geographic location and other) for which the measures were utilized were extracted. We extracted measures of surgical access disparities, as such, only studies which showed a statistically significant disparity in the specific surgical access measurement were included. This was done as the aim of this literature review was to extract measurable indicators in areas where significant disparities in surgical access were identified, these measurable indicators have the potential to be converted into disparity sensitive surgical access metrics. Studies were excluded if they were not written in English or not conducted in the US.
Data extraction and analysis
The individual measures of surgical access disparities were extracted from each study. Two investigators screened the articles and extracted the measures (EdJ and MG). For each measure, the reported disparity group was identified (racial/ethnic, education, insurance, income, geography and other). The measures were classified into surgical specialties by the 14 subspecialties defined by the American College of Surgeons; general, thoracic, colon and rectal, obstetrics and gynecology, gynecology oncology, neurology, ophthalmic, oral maxillofacial, orthopedic, otolaryngology, pediatric, plastic maxillofacial, urology and vascular.
Measures were categorized per a conceptual model derived from the Alkire et al, 2016 Global Access to Surgical Care model (10). The conceptual model was reviewed and refined in biweekly MEASUR project meetings with input from members of the American College of Surgeons, National Quality Forum, Eastern Virginia Medical School and the University of California—Los Angeles.
Measures were graphed on an evidence map; a systematic approach to evidence synthesis to represent gaps in knowledge and interpret individual studies within the context of a global knowledge on disparities to highlight future research needs (11). The evidence map illustrates the number of measures by surgical access domain (y-axis), surgical specialty (x-axis), disparity domain (color) and number of studies reporting disparities for each measure (diameter of the markers). This map depicts the landscape of evidence describing measures of surgical access disparities. Insights and findings from this map was derived through observation and discussion among co-authors and MEASUR project co-investigators.
Results
Search results
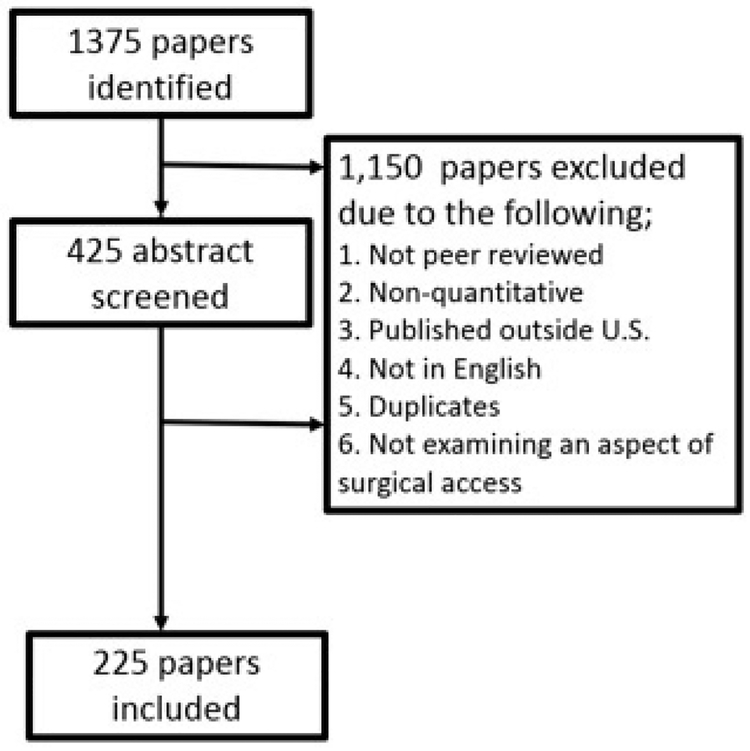
The search returned 1,375 original articles. Based on the inclusion and exclusion criteria, 225 studies were included. From these studies, 223 measures of surgical access were extracted (Fig 1).
Fig 1.
Flow diagram of studies for inclusion in a systematic literature review of measures of surgical access in the US.
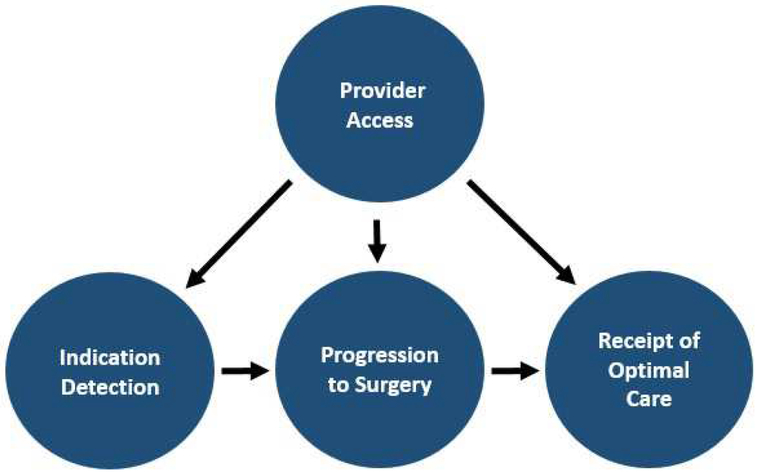
Conceptual Model of Surgical Access Disparities
Measures were classified based on the following categories: Provider Access, Surgical Indication Detection, Progression to Surgery and Receipt of Optimal Care (Fig 2). The unidirectional arrows in the diagram suggest how each of the categories sequentially influences other categories.
Fig 2.
A conceptual model for classifying surgical access disparity measures in the US.
Provider Access
Provider access measures reflect disparities in access to the highest quality of surgical care and discharge provider facilities. A total of 30 provider access measures were identified (Table 1). Measures showed disparities in the use of low-volume hospitals, low-volume surgical practitioners, the use of safety net hospitals, and the use of hospitals with a higher risk-adjusted surgical mortality. Key examples are described. One measure reported access disparities to a Level I or II trauma center within 60 minutes via an ambulance or helicopter (12). Three measures examined disparities in optimal discharge disposition to indicated rehabilitation facilities for trauma (13), cardiac surgery (14) and traumatic brain injury patients (15–17).
Table 1:
Provider Access Measures of Surgical Access Disparities
| Measure | Disparity group examined | Surgical specialty |
|---|---|---|
| Surgical access disparity | ||
| Proportion of patients with traumatic brain injury discharged to rehabilitation, long term acute care or nursing facilities | Racial/ethnic (15–17); insurance (15, 17) |
Neurological |
| Proportion of patients receiving trauma care who are discharged to an inpatient rehab facility | Other- undocumented immigrants(13) | General |
| Proportion of patients who are referred to cardiac rehabilitation post percutaneous intervention or cardiac surgery | Other- hospital specific (14) | Thoracic |
| Proportion of patients with access to a level 1 or 2 trauma center within 60 minutes via ambulance or helicopter | Income (12); geography (12); insurance (12) |
General |
| Disparity in the use of low volume hospitals/ low volume surgeon/safety net hospital use/hospital use with higher risk adjusted mortality | ||
| Hepatocellular carcinoma liver surgeries | Racial/ethnic (93); income (93); education (93) |
General |
| Pancreatic cancer resection | Racial/ethnic (94) | General |
| Knee arthroplasty | Racial/ethnic (95) | Orthopedic |
| Total hip replacement | Racial/ethnic (96) | Orthopedic |
| Thyroidectomy | Racial/ethnic (97, 98); Insurance (98); income (98) |
General |
| Coronary artery bypass grafting | Racial/ethnic (99) (100) | Thoracic |
| Adrenal operation | Racial/ethnic (101) | General |
| Endocrine surgery | Racial/ethnic (92); other - high health risk communities (92) |
General |
| Receiving radical prostatectomy at center offering robot-assisted radical prostatectomy | Racial/ethnic (102); income (102); insurance (102) |
Urology |
| Localized prostate cancer treatment by high volume urologist | Racial/ethnic (103) | Urology |
| Diagnosis by low volume and change to high volume for prostate cancer treatment | Racial/ethnic (103) | Urology |
| Colorectal cancer care | Racial/ethnic(104); insurance (104) |
Colon & rectal |
| Trauma surgery | Racial/ethnic(105) | General |
| Carotid endarterectomy | Racial/ethnic(41) | Vascular |
| Critical limb ischemia amputation or revascularization | Racial/ethnic (106); income (106); insurance (106) |
Vascular |
| Breast cancer resection for patients aged >65years | Racial/ethnic (75) | General |
| Pediatric neurooncological surgery | Racial/ethnic(107, 108); income (107) |
General |
| Brain tumor craniotomy | Racial/ethnic (42); insurance (42) |
Neurological |
| Breast cancer, colorectal cancer, gastric cancer, lung cancer, pancreatic cancer, coronary artery bypass grafting, angioplasty, abdominal aortic aneurysm repair, carotid endarterectomy, total hip replacement | Racial/ethnic (72) | General |
| Esophageal, pancreatic and colorectal cancer procedure | Racial/ethnic (109); insurance (109) |
General |
| Ovarian cancer surgical care | Racial/ethnic (110, 111) | Gynecologic oncology |
| Endometrial/uterine cancer | Racial/ethnic (112, 113) | Gynecologic oncology |
| Benign prostatic hypertrophy treatment access to hospitals offering laser prostatectomy | Income (114) | Urology |
| Scoliosis surgical procedure | Racial/ethnic (115) | Orthopedic |
| Grouped oncology- colectomy, cystectomy, esophagectomy, gastrectomy, hysterectomy, lung resection, pancreatectomy, prostatectomy | Racial/ethnic (116); income (116); insurance (116) |
General |
| Acute ischemic stroke admission to hospitals performing mechanical thrombectomy at high volume | Racial/ethnic (117); income (117); insurance (117) |
Neurological |
Surgical Indication Detection
Surgical indication detection measures represent disparities in the time of diagnosis, presentation or referral (detection) of a potential surgical condition (indication). There were 39 measures identified (Table 2). Twenty-one measures described a more advanced stage of cancer at the time of diagnosis including breast (18–22), lung (23), rectal (24), hepatocellular carcinoma (25) and pancreatic neuroendocrine tumors (26). Fourteen measures examined more advanced clinical presentations of conditions (meaning non-cancer). Examples of advanced clinical presentations include the severity (patency) of peripheral arterial disease at presentation (27–31), the time from onset of pediatric abdominal pain to presentation for appendicitis (32) and pain intensity or wellbeing scores for patients undergoing knee replacements for osteoarthritis (33).
Table 2:
Surgical Indication Detection Measures of Surgical Access Disparities
| Measure of surgical access disparity | Disparity group examined | Surgical specialty | |
|---|---|---|---|
| Stage of diagnosis for pediatric patients aged ≤ 21 diagnosed with primary sarcomas | Racial/ethnic (118) | Pediatric | |
| Stage of diagnosis for pediatric patients diagnosed with well differentiated thyroid cancer | Income (119); education (119); insurance (119) |
Pediatric | |
| Stage at diagnosis of pediatric patients aged ≤ 10 years diagnosed with melanoma | Racial/ethnic (120) | Pediatric | |
| Stage at presentation of pediatric patients aged ≤ 18 years diagnosed with primary central nervous system tumors | Racial/ethnic (121) | Pediatric | |
| Stage of diagnosis for patients diagnosed with a pancreatic neuroendocrine tumor | Racial/ethnic (26) | Pediatric | |
| Stage of diagnosis at presentation of patients diagnosed with adenocarcinomas | Racial/ethnic (122); geography (122) |
General | |
| Stage of diagnosis for patients diagnosed with rectal cancer | Insurance (24) | Colon & rectal | |
| Stage at diagnosis of patients with endometrial cancer | Racial/ethnic (112, 123, 124) | Gynecologic oncology | |
| Stage at diagnosis of patients with cervical cancer | Insurance (125); racial/ethnic (126, 127) |
Gynecologic oncology | |
| Stage at diagnosis of patients with breast, prostate or colorectal cancer | Income (128) education (128) |
General | |
| Stage at diagnosis of patients with breast cancer (female) | Racial/ethnic (18, 19); income (18–20); geography (20) ; insurance (21) |
General | |
| Stage at diagnosis of patients with breast cancer (male) | Income (22) | General | |
| Proportion of patients with metastatic disease at the time of diagnosis of small bowel carcinoid tumor | Racial/ethnic (129) | General | |
| Stage at diagnosis of patients with soft tissue sarcoma (in the extremity) | Racial/ethnic (130) | Orthopedic | |
| Proportion of patients with well differentiated thyroid cancer detected incidentally on unrelated imaging | Insurance (131) | General | |
| Stage of tumor disease severity at presentation for patients with brain tumors | Racial/ethnic (42); insurance (42) |
Neurological | |
| Stage at diagnosis of patients with hepatocellular carcinoma | Racial/ethnic (25); insurance (25) |
General | |
| Stage at diagnosis of patients with lung cancer | Racial/ethnic (23) | Thoracic | |
| Stage at diagnosis of patients with vestibular schwannoma | Racial/ethnic (132) | Neurological | |
| Later stage of diagnosis for patients diagnosed with prostate cancer measured by prostate specific antigen (psa) level or gleason scores | Racial/ethnic (133) | Urology | |
| Proportion of patients with chronic venous insufficiency presenting with advanced disease requiring ulcer debridement | Racial/ethnic (134) | Vascular | |
| Clinical severity score in venous insufficiency patients presenting for radiofrequency ablation | Racial/ethnic (135) | Vascular | |
| Proportion of patients with peripheral artery disease presenting with critical limb ischemia | Racial/ethnic (136) | Vascular | |
| Proportion of peripheral artery disease presenting with critical limb ischemia who undergo a revascularization attempt (limb salvage) vs amputation | Racial/ethnic (27, 137) | Vascular | |
| Severity of patients with peripheral artery disease at presentation (patency) | Racial/ethnic (27–29); insurance (28, 29, 31); income (29, 30) |
Vascular | |
| Rate of limb salvage in patients presenting with advanced peripheral arterial disease | Racial/ethnic (27, 29, 138); insurance (29, 31); income (29, 30) |
Vascular | |
| Proportion of patients undergoing carotid endarterectomy for asymptomatic carotid artery stenosis | Insurance (139) ; racial/ethnic (139) |
Vascular | |
| Age at first presentation of patients presenting with pediatric nonsyndromic craniosynotosis | Racial/ethnic (140) | Pediatric | |
| Age at first surgical intervention for patients presenting with pediatric nonsyndromic craniosynotosis | Insurance (141); racial/ethnic (141) |
Pediatric | |
| Proportion of pediatric patients with perforated appendicitis | Racial/ethnic (32, 46); income (32, 46); Insurance (32) |
Pediatric | |
| Proportion of pediatric appendicitis patients experiencing symptoms > 48 hours before surgical presentation | Racial/ethnic (32) | General | |
| Proportion of patients with perforated appendicitis | Insurance (142) | General | |
| Pre-operative knee replacement wellbeing scale | Racial/ethnic (33) | Orthopedic | |
| Pre-operative knee replacement pain intensity scale | Racial/ethnic (33) | Orthopedic | |
| Complexity score of presenting problems at a tertiary care hand surgery facility (higher) | Insurance (143) | Orthopedic | |
Progression to Surgery
Progression to surgery measures reflect disparities in the process of attaining a surgical opinion or procedure once a surgical indication has been detected. One hundred measures describing progression to surgery were identified (Table 3). Four measures examined disparities in persons for whom surgery was indicated being offered a surgical option for conditions like pelvic organ prolapse (34), loco-regional pancreatic cancer (35), or pediatric sensorineural hearing loss (36). Thirty-six measures examined disparities in persons with a potential surgical indication receiving the indicated surgical procedure (decision to treat) for cancer, vascular, orthopedic, cardiac, neurosurgery, otolaryngology, and bariatric surgery. Twelve measures examined disparities in the total surgical rates per population for surgeries like joint replacements (37–39), elective abdominal aortic aneurysm repair (40), or carotid endarterectomies (41). Five measures examined disparities in the emergency-to-elective procedure ratios for conditions in which an elective procedure may have prevented a later emergency procedure, like abdominal aortic aneurysm repair (40) or brain tumor craniotomies (42). Disparities in the time between surgical indication detection and the surgical evaluation or procedure were examined by eight measures. Examples of this include the time between a breast cancer biopsy to surgery (43–45), the time between presentation of pediatric abdominal pain to undergoing appendectomy (46) and the interval between sonographic detection of carotid stenosis warranting carotid endarterectomy and the procedure (47).
Table 3:
Progression to surgery measures of surgical access disparities.
| Measure of surgical access disparity | Disparity group examined | Surgical specialty |
|---|---|---|
| Proportion of women with pelvic organ prolapse offered a surgical option | Income (34); insurance (34) |
Obstetrics & gynecology |
| Proportion of patients with advanced knee/hip replacement being recommended for a total joint replacement | Racial/ethnic (144) | Orthopedic |
| Proportion of patients with sensorineural hearing loss meeting the audiology criteria for cochlear implantation receiving pediatric cochlear implant referral | Insurance (36); other -married parents (36) |
Otolaryngology |
| Proportion of patients with hepatocellular carcinoma receiving a surgical referral | Geography (145); insurance (145) |
General |
| Proportion of patients with locoregional pancreatic cancer receiving a surgical consult or evaluation | Racial/ethnic (35) | General |
| Cumulative proportion of patients with breast, prostate, lung or colorectal cancer, who undergo indicated surgical treatment | Income (128); education (128) |
General |
| Cumulative proportion of patients who receive indicated first course directed surgery for breast, prostate, lung, uterine, cervix, ovarian, melanoma, urinary bladder and colorectal cancer | Racial/ethnic (146) | General |
| Proportion of patients who receive all indicated treatment for breast, prostate, lung, uterine, cervix, upper gi, head & neck cancer, hodgkin lymphoma and diffuse b cell lymphoma | Other -hiv patients (147) | General |
| Proportion of patients who undergo indicated pancreatic cancer surgical resection | Insurance (90, 122); racial/ethnic (35, 122); Geography (122) |
General |
| Proportion of patients who undergo indicated pancreatic neuroendocrine tumor surgical resection | Racial/ethnic (26) | General |
| Proportion of patients who undergo indicated pancreatic adenocarcinoma surgical resection | Racial/ethnic (148) ; income(149) |
General |
| Proportion of patients who undergo indicated surgery for uterine grade iii endometrial adenocarcinoma, carcinosarcoma, clear cell carcinoma & papillary serous carcinoma | Racial/ethnic (123) | Gynecologic oncology |
| Proportion of patients who undergo definitive surgery for high grade endometrial cancer | Racial/ethnic (150) | Gynecologic oncology |
| Proportion of patients who undergo definitive surgery for cervical cancer | Insurance (125) | Gynecologic oncology |
| Proportion of patients who undergo stage adjusted surgery for cervical cancer | Racial/ethnic (127) | Gynecologic oncology |
| Proportion of patients who undergo indicated prostate cancer resection | Racial/ethnic (133, 151); insurance (151); income (151) |
Urology |
| Proportion of patients undergoing surgical resection for lung cancer | Racial/ethnic (59, 152) | Thoracic |
| Proportion of stage adjusted patients undergoing surgical resection for lung cancer | Racial/ethnic (59) | Thoracic |
| Proportion of patients undergoing surgery for localized non-small cell lung cancer | Racial/ethnic (153, 154) | Thoracic |
| Proportion of patients undergoing surgical resection for stage i and ii non small cell lung cancer within 6 weeks of diagnosis | Racial/ethnic (155) | Thoracic |
| Proportion of patients undergoing surgical resection, chemotherapy or radiation for stage iii non small cell lung cancer within 6 weeks of diagnosis | Racial/ethnic (155) | Thoracic |
| Proportion of patients undergoing surgical resection for stage i and ii non small cell lung cancer | Racial/ethnic (156) | Thoracic |
| Proportion of patients with lung cancer receiving the ‘standard’ therapy | Insurance (157) | Thoracic |
| Proportion of patients undergoing surgical resection for breast cancer | Income (20, 73); geography (20) ; racial/ethnic (43, 73) |
General |
| Proportion of patients undergoing surgical resection for non-metastatic breast cancer | Racial/ethnic (74) | General |
| Proportion of patients aged over 65 years who receive primary surgical treatment (mastectomy or breast conserving surgery) for stage i and ii breast cancer | Racial/ethnic (75) | General |
| Proportion of patients who receive stage specific treatment for breast cancer | Insurance (71) | General |
| Proportion of patients undergoing surgery for colorectal cancer | Racial/ethnic (73); income (73); Insurance (158) |
Colon & rectal |
| Proportion of patients undergoing definitive surgery for rectal cancer | Insurance (24) | Colon & rectal |
| Proportion of patients older than 80 years undergoing surgery for colorectal cancer | Racial/ethnic (159) | Colon & rectal |
| Proportion of hiv patients who receive indicated cancer treatment for cumulative solid tumor and lymphoma | Racial/ethnic (147); insurance (147) |
General |
| Proportion of patients undergoing debulking surgery for ovarian cancer | Income (160) | Obstetrics & gynecology |
| Proportion surgery for ovarian cancer | Racial/ethnic (160, 161) | Obstetrics & gynecology |
| Proportion of patients undergoing surgery for soft tissue sarcoma of the extremities | Racial/ethnic (130) | Orthopedic |
| Proportion of patients undergoing hepatectomy or ablation for hepatocellular carcinoma | Racial/ethnic (162) | General |
| Proportion of patients undergoing esophagectomoy for esophageal cancer | Racial/ethnic (163) | General |
| Proportion of patients undergoing surgery for vestibular schwannomas | Racial/ethnic (132) | Neurological |
| Proportion of patients with peripheral arterial disease meeting vascular lab criteria for procedural intervention receiving the intervention (angioplasty, stenting, endarterectomy or bypass grafting) | Racial/ethnic (79) | Vascular |
| Proportion of patients with carotid artery disease meeting vascular lab criteria for intervention undergoing a procedure [carotid end arterectomy or stenting] | Racial/ethnic (79) insurance (164) |
Vascular |
| Proportion of patients undergoing procedure when admitted for a traumatic brain injury | Insurance (15) | Neurological |
| Proportion of patients undergoing mechanical revascularization procedures after acute ischemic stroke | Racial/ethnic (117); income (117); insurance (117) |
Neurological |
| Proportion of elderly patients with arteriovenous fistulas among hemodialysis patients | Racial/ethnic (165) | Vascular |
| Proportion of patients undergoing total joint replacement for advanced knee/hip osteoarthritis | Racial/ethnic (144, 166); income (166) |
Orthopedic |
| Proportion of patients undergoing knee replacement for knee osteoarthritis | Education (167); insurance (167) |
Orthopedic |
| Proportion of patients undergoing hip replacement for hip osteoarthritis | Education (167); insurance (167) |
Orthopedic |
| Proportion of patients with scoliosis undergoing surgical intervention | Insurance (115); racial/ethnic (115) |
Orthopedic |
| Proportion of patients undergoing catheter ablation for atrial fibrillation | Racial/ethnic (168); insurance (168) |
Thoracic |
| Proportion of patients undergoing a procedure (angiogram, per cutaneous intervention, coronary artery bypass grafting) during/after acute myocardial infarction admission | Racial/ethnic (169); sex (169) |
Thoracic |
| Proportion of patients undergoing coronary revascularization (coronary artery bypass grafting or percutaneous coronary intervention) after myocardial infarction | Racial/ethnic (170); education (170); insurance (170) |
Thoracic |
| Proportion of patients undergoing aortic valve replacement for severe aortic stenosis | Racial/ethnic (171) | Thoracic |
| Proportion of patients declining indicated aortic valve replacement recommendation for severe aortic stenosis | Racial/ethnic (171) | Thoracic |
| Proportion of patients receiving indicated bariatric surgery | Racial/ethnic (172, 173); geography (172); income (173, 174); insurance (173) |
General |
| Proportion of patients receiving bariatric surgery, laparoscopic gastric bypass, when indicated | Racial/ethnic (175) | General |
| Proportion of patients undergoing tympanostomy tube placement for otitis media | Racial/ethnic (176) | Otolaryngology |
| Proportion of medicare patients undergoing deep brain simulation surgery for parkinson’s disease | Racial/ethnic (177); income (177) |
Neurological |
| Time between diagnosis of pediatric well-differentiated thyroid cancer to intervention | Income (119); insurance (119) |
General |
| Time between diagnosis of gynecological malignancy to surgery | Other - public vs. Private hospital (178) | Gynecologic oncology |
| Time between presentation of pediatric abdominal pain to appendectomy | Racial/ethnic (46); income (46) |
Pediatric |
| Time between diagnosis via sonogram of carotid stenosis warranting carotid endarterectomy and carotid endarterectomy receipt | Racial/ethnic (47) | Vascular |
| Time between clinical presentation of ureteropelvic junction obstruction to urology evaluation/pyeloplasty | Racial/ethnic (179) | Urology |
| Time between abnormal imaging or core biopsy to surgery for breast cancer patients | Racial/ethnic (43–45); insurance (44) |
General |
| Time from polysomnography test recommending adenotonsilectomy for children with sleep disordered breathing to receipt of adenotonsilectomy among pediatric patients | Insurance (180) | Otolaryngology |
| Proportion of adults undergoing knee arthroplasty | Racial/ethnic (37, 38) | Orthopedic |
| Proportion of adults over 65 undergoing knee/hip arthroplasty | Racial/ethnic (39) | Orthopedic |
| Proportion of patients with peripheral vascular disease receiving lower extremity amputation | Racial/ethnic (181) | Vascular |
| Proportion of patients undergoing an elective abdominal aortic aneurysm repair per population | Racial/ethnic (40) | Vascular |
| Proportion of patients receiving a thoracic endovascular aneurysm repair per population | Racial/ethnic (182) | Vascular |
| Proportion of people receiving stress urinary incontinence surgery per population | Racial/ethnic (183) | Vascular |
| Proportion of people receiving carotid endarterectomy per population | Racial/ethnic (41) | Vascular |
| Outpatient surgery volume per population | Racial/ethnic (184) | General |
| Number of general surgeons per population | Racial/ethnic (184) | General |
| Number of carotid endarterectomy, lumbar spine fusion, knee replacement, aa repair, prostatectomy, hip replacement, aortic valve repair, open & internal fixation of the femur and appendectomy procedures per population | Geography (185) | General |
| Number of laparoscopic appendectomy procedures per population | Racial/ethnic (186); income (186) |
General |
| Proportion of patients with diabetes hospitalized for diabetes-related cardiovascular disease who receive a cardiac procedure (percutaneous transluminal coronary angioplasty, coronary artery bypass grafting) | Racial/ethnic (187) | Thoracic |
| Proportion of patients undergoing angiography and revascularization procedures after myocardial infarction | Racial/ethnic (188); income (188) |
Thoracic |
| Proportion of patients with lumbar spine stenosis who undergo surgery | Racial/ethnic (189) | Orthopedic |
| Ratio of emergency to elective gastrointestinal procedures among pediatric patients | Racial/ethnic (190) | Pediatric |
| Ratio of emergency to elective abdominal aortic aneurysm procedures | Racial/ethnic (40) | Vascular |
| Ratio of emergency to elective thoracoabdominal aortic aneurysm procedures | Racial/ethnic (191) | Vascular |
| Ratio of emergency to elective craniectomy procedures for brain tumors | Racial/ethnic (42); insurance (42) |
Neurological |
| Ratio of cumulative emergency to elective biliary, hernia and colorectal operations | Racial/ethnic (192) | General |
| Proportion of patients with early hepatocellular carcinoma who receive a liver transplant | Racial/ethnic (25, 162, 193–195); Insurance (25, 193, 194) |
General |
| Proportion of patients with hepatocellular carcinoma receiving surgery (local ablation or a liver transplant) | Income (162); insurance (162) |
General |
| Proportion of patients not listed on hepatocellular carcinoma liver transplant list for non-medical reasons | Insurance (194); racial/ethnic (194) |
General |
| Proportion of patients placed on the liver transplant list for end stage liver disease | Education (196); insurance (196); racial/ethnic (196) |
General |
| Proportion of patients with end stage liver disease referred to a transplant center | Racial/ethnic (196) | General |
| Proportion of patients who attend a transplant center if referred for end stage liver disease | Racial/ethnic (196) | General |
| Higher model for end-stage liver disease (meld) score on liver transplant waitlist | Racial/ethnic (197) | General |
| Proportion of patients receiving a liver transplant within 3 years of being listed | Racial/ethnic (197) | General |
| Higher model for end-stage liver disease (meld) score at presentation to a liver transplant center | Racial/ethnic (198) | General |
| Proportion of patients referred early for liver transplant evaluation | Racial/ethnic (198) | General |
| Proportion of patients on the active renal transplant waitlist | Other- linguistic isolation (199) | General |
| Proportion of patients who are referred for a renal transplant | Income (91) | General |
| Proportion of patients who die whilst on renal transplant waitlist | Income (200) | General |
| Proportion of patients on the renal transplant waitlist who received a renal transplant | Income (200) ; racial/ethnic (201) |
General |
| Proportion of patients who attend/present to their first renal transplant evaluation appointment | Racial/ethnic (201) | General |
| Proportion of patients on the renal transplant waitlist who are inactive due to loss of follow up | Racial/ethnic (201) | General |
| Median time from transplant referral to decreased donor transplant | Racial/ethnic (201) | General |
| Time from a patient starting renal dialysis to being placed on the renal transplant waitlist | Insurance (202); income (202); racial/ethnic (202) |
General |
| Proportion of patients who receive a preemptive (pre-dialysis initiation) decreased donor renal transplant | Insurance (203); racial/ethnic (203) |
General |
| Proportion of patients who receive assessment for renal transplantation | Insurance (204); racial/ethnic (204) |
General |
| Proportion of pediatrics patients who receive a preemptive renal live donor transplant | Racial/ethnic (205) | General |
| Proportion of patients with cystic fibrosis who are accepted onto the lung transplant waitlist after their first lung transplant evaluation | Insurance (206); education (206); income (206) |
General |
Receipt of Optimal Care
Receipt of optimal care measures reflect disparities in a patient’s ability to receive the highest quality surgical care. A total of 54 measures examined optimal care disparities, 37 examined surgical care and 14 examined postoperative follow up care (Table 4). These measures included disparities in post-mastectomy breast reconstruction (48–57), the recommended number of lymph nodes removed for conditions like gastric cancer (58) or lung cancer (59), immediate versus delayed cholecystectomy rates (60), and amputation rates for lower extremity open fractures (61). Fifteen measures examined disparities in the utilization of minimally invasive procedures such as laparoscopic appendectomies (62), hysterectomies (63, 64) or cholecystectomies (65), as well as breast-conserving surgery versus mastectomies (18, 19, 21, 66–68). Optimal postoperative follow-up measures examined disparities in emergency stoma reversal rates (69), rates of breast-conserving surgery without receipt of full radiotherapy (43), or failure to complete internal fixation removal for cases of pediatric femoral shaft fractures (70).
Table 4:
Receipt of Optimal Care Measures of Surgical Access Disparities
| Measure of surgical access disparity | Disparity group examined | Surgical specialty |
|---|---|---|
| Proportion of early-stage, unilateral breast cancer patients receiving breast reconstruction after mastectomy | Racial/ethnic (48–56); insurance (48, 50, 52, 54–56) ; geography (48, 57); income (50, 54); education (50, 51) |
General |
| Proportion of patients receiving breast reconstruction during the same hospitalization (i.e. immediately) after mastectomy | Insurance (152, 207); racial/ethnic (77, 78); income (77); education (77) |
General |
| Proportion of stage 0 to ii unilateral breast cancer patients receiving contralateral prophylactic mastectomy | Racial/ethnic (208) | General |
| Ratio of mastectomies performed in outpatient vs. Inpatient settings | Racial/ethnic (209) | General |
| Proportion of patients with hepatocellular carcinoma who undergo bridging locoregional therapy among all patients who receive an orthotropic liver transplant | Racial/ethnic (210); education (210); insurance (210) |
General |
| Proportion of patients with soft tissue sarcoma who receive limb sparing surgery | Racial/ethnic (211, 212) | Orthopedic |
| Proportion of patients with ovarian cancer undergoing bowel resection, peritoneal biopsy/omentectomy | Racial/ethnic (111) | Gynecologic oncology |
| Proportion of gastric cancer patients with surgically treated gi malignancy receiving “adequate lymphadenectomy” (more than 15 esophagus, 15 stomach, 12 small bowel, 12 colon, 12 rectum, and 15 pancreas) | Income (58) | General |
| Proportion of lung cancer patients receiving appropriate lymph node resection | Racial/ethnic (59) | Thoracic |
| Stage-adjusted proportion of lung cancer patients receiving appropriate lymph node resection | Racial/ethnic (59) | Thoracic |
| Proportion of ovarian cancer patients who have the recommended number of lymph nodes removed | Racial/ethnic (111) | Gynecologic oncology |
| Proportion of breast cancer patients who have the recommended number of lymph nodes removed | Racial/ethnic (76) | General |
| Proportion of patients with gastric cancer who have the recommended number of lymph nodes removed | Insurance (213) | General |
| Proportion of patients localized/regional prostate cancer who have the recommended number of lymph nodes removed | Racial/ethnic (214) | Urology |
| Proportion of pediatric patients presenting to the emergency department with abdominal pain who received abdominal ct imaging to confirm appendicitis | Racial/ethnic (46) ; insurance (46) |
General |
| Proportion of patients with end-stage renal disease who have an arteriovenous fistula at initial hemodialysis | Racial/ethnic (215) | Vascular |
| Proportion of patients with acute cholecystitis who receive immediate cholecystectomy | Insurance (60) ; racial/ethnic (60) |
General |
| Proportion of all patients admitted for acute ischemic stroke who receive reperfusion on the first admission day, invasive angiography, and operative procedures including carotid endarterectomy | Income (216) | Neurological |
| Proportion of all carotid endarterectomy surgeries with inappropriate clinical indicators (high comorbidity in asymptomatic patient, operating in a setting of a recent or severe disabling stroke, minimal stenosis, operating contralateral to symptoms, occluded artery) | Racial/ethnic (41) | Vascular |
| Proportion of patients with lower extremity fractures (open tibial/fibular and femoral fractures) who undergo amputations | Racial/ethnic (61) | Orthopedic |
| Proportion of patients with graves’ disease who receive a thyroidectomy | Income (217) | General |
| Proportion of patients presenting with blunt injuries with pelvic fractures who receive diagnostic procedures (vascular ultrasound, ct of the abdomen), transfusions, venous pressure monitoring, and arterial catheterization for embolization | Insurance (218) | General |
| Proportion of patients with symptomatic heart failure who receive biventricular pacing | Racial/ethnic (219); income (219); insurance (219) |
Thoracic |
| Proportion of patients undergoing major elective orthopedic surgery who receive autologous blood transfusion | Insurance (220); income (220); racial/ethnic (220) |
Orthopedic |
| Proportion of patients receiving laparoscopic vs. Open hysterectomies | Racial/ethnic (63) | Obstetrics & gynecology |
| Proportion of patients receiving laparoscopic vs. Open hysterectomies for the indication of fibroids | Racial/ethnic (64) | Obstetrics & gynecology |
| Proportion of patients receiving laparoscopic vs. Open hysterectomy for uterine cancer | Racial/ethnic (113) | Gynecologic oncology |
| Proportion of breast cancer patients who receive breast conservation surgery vs. Mastectomy | Insurance (18, 21); racial/ethnic (19, 66, 67); income (66, 68) |
General |
| Proportion of cumulative open vs. Laparoscopic rates of cumulative colorectal surgery for colorectal cancer, diverticular disease, inflammatory bowel disease and benign colorectal tumors | Racial/ethnic (221) | Colon & rectal |
| Proportion of patients undergoing surgery for colorectal cancer who have laparoscopic vs. Open surgery | Income (222) ; insurance (222, 223); geography (223) |
Colon & rectal |
| Proportion of patients undergoing surgery for ulcerative colitis open vs. Laparoscopic surgery | Insurance (224) | General |
| Proportion of patients undergoing a laparoscopic vs. Open surgery appendectomy, gastric fundoplication or gastric bypass | Racial/ethnic (62) | General |
| Proportion of patients undergoing acute surgery, minimally invasive vs. Open for appendectomy or cholecystectomy | Racial/ethnic (65); insurance (65) |
General |
| Rates of laparoscopic vs. Open appendectomies for patients ages between 11 and 18. | Racial/ethnic (225) | Pediatric |
| Proportion of patients undergoing an abdominal aortic aneurysm repair via endovascular vs. Open surgery | Racial/ethnic (226, 227); insurance (226) |
Vascular |
| Proportion of patients undergoing a thoracic aortic repair via thoracic endovascular aortic repair vs. Open surgery | Racial/ethnic (228); | Vascular |
| Proportion of patients presenting with critical limb ischemia from peripheral arterial disease undergoing endovascular or open revascularization vs. Amputation | Racial/ethnic (106, 229); income (106); insurance (106) |
Vascular |
| Proportion of patients receiving a non-traumatic amputation as the transfermoral compared to transtibial position | Income (230); insurance (230); Other- sex (230) |
Orthopedic |
| Proportion of patients with choledocholithiasis receiving ercp vs. Common bile duct exploration | Geography (231) | General |
| Proportion of patients undergoing a radical prostatectomy vs. A minimally invasive radical prostatectomy | Racial/ethnic (232) | Urology |
| Proportion of patients who underwent a breast conserving surgery for breast cancer completing the recommended length of radiation | Insurance (18) ; racial/ethnic (43) |
General |
| Proportion of patients post mastectomy for local-regionally advanced breast cancer receiving local radiotherapy | Geography (233) | General |
| Proportion of patients post endometrial cancer surgery completing the recommended adjuvant radiotherapy/chemotherapy course | Insurance (124) | Gynecologic oncology |
| Proportion of women receiving surgery for ovarian cancer who receive the recommended adjuvant chemotherapy post operatively | Income (160) | Gynecologic oncology |
| Proportion of patients discharged post lower limb trauma admission who receive follow up inpatient care | Insurance (234) | Orthopedic |
| Proportion of patients with cervical cancer who undergo surgery and do not receive the recommended postoperative radiotherapy | Insurance (125) | Gynecologic oncology |
| Proportion of patients who receive a stoma reversal | Racial/ethnic (69) ; insurance (69) ; income (69) |
General |
| Proportion of patients receiving post hospital trauma care | Insurance (235) | General |
| Proportion of people with a colon cancer colectomy who receive the recommended adjuvant chemotherapy | Geography (236) | Colon & rectal |
| Proportion of patients with primary extremity soft tissue sarcoma who receive adjuvant radiation | Racial/ethnic (211) | Orthopedic |
| Proportion of patient who have femoral shaft internal fixation materials removed | Racial/ethnic (70); Income (70) |
Orthopedic |
| Proportion of prelingual patients who receive a cochlear implant undergoing a sequential cochlear implantation on the other side | Insurance (237) | Otolaryngology |
| Proportion of patients who miss a follow up appointment post cochlear implantation | Insurance (237) | Otolaryngology |
| Proportion of patients who fail to arrive at an appointment at a tertiary hand surgery referral center | Insurance (143) | Orthopedic |
Disparity groups
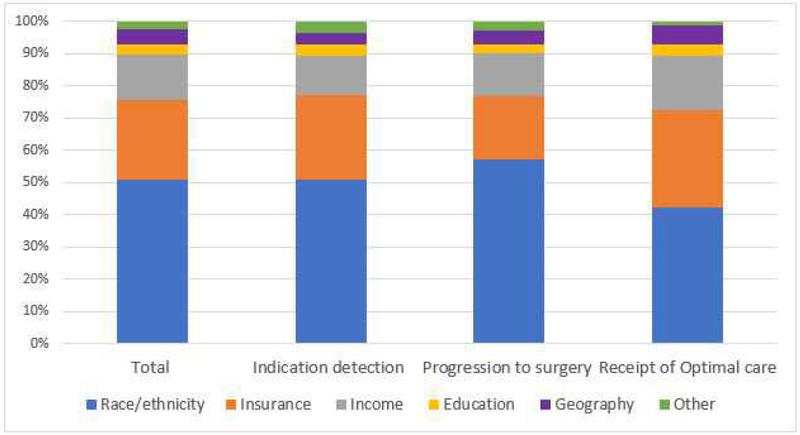
The measures of surgical access were categorized into six disparity domains; racial/ethnic, education, insurance, income, geography, and other (e.g. marital status, sex, immigration status). Figure 3 shows that the proportion of measures in each group remained similar throughout the phases of the conceptual surgical access model.
Fig 3.
Measures of surgical access disparities in each surgical access segment categorized by disparity domain (race/ethnicity, insurance. income. education, geography, and other).
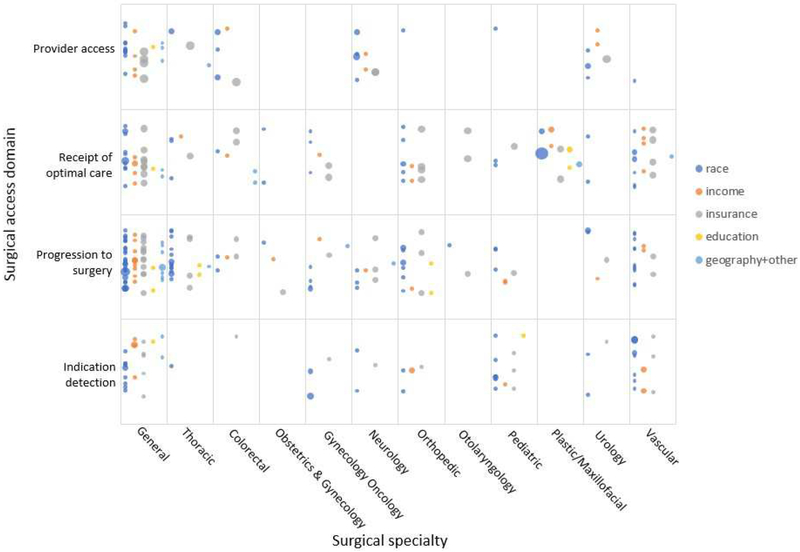
Evidence map
An evidence map illustrating the 223 measures of surgical access disparities in the US, stratified by surgical access domain, surgical specialty and disparity domain is shown in Figure 4. Two surgical specialties (ophthalmic and oral maxillofacial) did not have any measures of disparities identified and were thus not included in the evidence map.
Fig 4.
Evidence map of measures of surgical access disparities in the US. *Bubble size indicates number of studies supporting each measure. Plotting of the bubbles in each cell is systematic to increase readability of the figure. Horizontally, the bubbles are in five rows based on the disparity domain (color). A random placement generator was used to distribute bubbles vertically inside each cell.
Discussion
Measures of surgical access disparities in the US have been broadly applied; our novel conceptual model, illustrates the multiple interrelated strata of disparities. By further compartmentalizing surgical access disparities, more targeted interventions can be developed to accurately measure and address them.
For breast cancer surgical care, as an example, there are various measures in each surgical access domain. Racial/ethnic minority patients are more likely to access care at lower quality providers (71, 72), the indication for surgery is detected later (present at a later stage of diagnosis (18, 19)), progression to surgery takes longer (increased biopsy diagnosis to surgery time (43–45)), and indicated surgical treatment is less likely to occur (43, 73–75). If a surgical procedure is performed, it may not be the optimal procedure for the patient and the patient may not receive the adequate follow-up (less breast-conserving surgery vs. mastectomy (19, 66, 67), less likely to receive the full indicated postoperative radiotherapy course (43), less likely to have recommended sentinel lymph node biopsies (76), lower post-mastectomy breast reconstruction rates (48–56, 77, 78)). This can be simplified to say that racial/ethnic minority populations have less access to breast cancer surgical care, but by addressing each domain, a more thorough understanding is developed.
Each disparity measure in each facet of the surgical access model may have a multitude of causal factors. Etiologies contributing to disparities in surgical access include; healthcare literacy, ability to navigate the healthcare system, mistrust of healthcare providers and hospitals, healthcare affordability, misunderstanding of disease severity and treatment options, and a lack of access to adequate health care facilities and qualified personnel (8, 79–83).
Another factor to consider is implicit bias in the healthcare system towards minority groups (84, 85). Implicit bias is defined as a ubiquitous societal preference for a social group that is both unconscious and automatic informed by an individual’s experiences and perceptions of others (86). Physician implicit bias may negatively impact patient communication, clinical assessments, and decision-making for vulnerable patients (87–89).
The disparity domains examined are co-linear. Race/ethnicity and the various social determinants of health are intricately linked. Some studies adjust for the confounding effects of each determinant. While this allows a category such as race/ethnicity to be examined individually, it may not reflect the true extent of the ‘real-life’ disparity for these groups.
Most of the studies examining surgical access disparities utilized large existing retrospective databases. The disparity domains examined are thus limited to the availability of these variables in the databases themselves. The disparity domain for measures of surgical access disparities were predominately race/ethnicity followed by insurance status and income. Few studies examined other disparity domains such as HIV status (90), immigration status (14), linguistic isolation (91) and residing in a high health risk community (92).
Limitations of this review include that all measures were stratified into one surgical specialty and surgical access domain. Some cumulative disparity measures may include more than one surgical specialty. These were largely classified as general – this may skew the evidence map displaying access disparities towards general surgery. We included only studies quantitatively comparing surgical access measures which found a significant disparity. As such, if the evidence map does not show a measure in each area, it is not known whether this is because there is no research in this area or if there is literature that did not find a significant disparity.
The evidence map illustrates areas for future targeted quality improvement intervention and areas where more research is needed. Cells populated with many measures are areas where disparity improvement initiatives may be targeted. An example of a populated cell is the surgical indication detection domain in vascular surgery. Measures in this cell are largely delays in presentation of peripheral arterial disease or abdominal aortic aneurysms, presentation delays may result in emergent and/or more invasive surgical procedures. Quality improvement efforts to reduce disparities in this area may be warranted. The evidence map also illustrates cells with few or no measures, some of these cells are in surgical fields where there are known disparities in surgical outcomes. These are areas where further health outcomes research examining disparities are warranted.
Conclusion
Two hundred and twenty-three measures of surgical access disparities in the US are available. These measures were categorized using a four-faceted conceptual model for surgical access; Provider Access, Surgical Indication Detection, Progression to Surgery and Receipt of Optimal Care. This model establishes a novel paradigm for conceptualizing surgical access disparities. These measures were illustrated in an evidence map which displays many critical gaps in the literature. It is essential to incorporate measures of surgical access disparities into future surgical improvement initiatives.
Acknowledgments
Support: This study was supported by the National Institute on Minority Health and Health Disparities of the National Institute of Health under award number: 5RO1MD011695–02. Elzerie de Jager is a Doctor of Philosophy candidate at an Australian university, this research degree is supported by an Australian Government Research Training Program (RTP) Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
References
- 1.Fiscella K, Franks P, Doescher MP, Saver BG. Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample. Med Care. 2002;40:52–9. [DOI] [PubMed] [Google Scholar]
- 2.Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216:482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torain MJ, Maragh-Bass AC, Dankwa-Mullen I, et al. Surgical Disparities: A Comprehensive Review and New Conceptual Framework. J Am Coll Surg. 2016;223:408–18. [DOI] [PubMed] [Google Scholar]
- 4.Schoenfeld AJ, Belmont PJ Jr., See AA, et al. Patient demographics, insurance status, race, and ethnicity as predictors of morbidity and mortality after spine trauma: a study using the National Trauma Data Bank. Spine J. 2013:1766–73. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen GC, Laveist TA, Segev DL, Thuluvath PJ. Race is a predictor of in-hospital mortality after cholecystectomy, especially in those with portal hypertension. Clin Gastroenterol Hepatol. 2008;6:1146–54. [DOI] [PubMed] [Google Scholar]
- 6.Reames BN, Birkmeyer NJ, Dimick JB, Ghaferi AA. Socioeconomic disparities in mortality after cancer surgery: failure to rescue. JAMA Surg. 2014;149:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In: Millman M, ed. Access to Health Care in America. Washington (DC); 1993. [PubMed] [Google Scholar]
- 8.Ayanian JZ. Determinants of racial and ethnic disparities in surgical care. World J Surg. 2008;32:509–15. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg JM, Power EJ. Transforming insurance coverage into quality health care: voltage drops from potential to delivered quality. JAMA. 2000;284:2100–7. [DOI] [PubMed] [Google Scholar]
- 10.Alkire BC, Raykar NP, Shrime MG, et al. Global access to surgical care: a modelling study. Lancet Glob Health. 2015;3:e316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr BG, Bowman AJ, Wolff CS, et al. Disparities in access to trauma care in the United States: A population-based analysis. Injury. 2017;48:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyrick JM, Kalosza BA, Coritsidis GN, Tse R, Agriantonis G. Trauma care in a multiethnic population: effects of being undocumented. The J Surg Res. 2017;214:145–53. [DOI] [PubMed] [Google Scholar]
- 14.Beatty AL, Bradley SM, Maynard C, McCabe JM. Referral to Cardiac Rehabilitation After Percutaneous Coronary Intervention, Coronary Artery Bypass Surgery, and Valve Surgery: Data From the Clinical Outcomes Assessment Program. Circ Cardiovasc Qual Outcomes. 2017;10. [DOI] [PubMed] [Google Scholar]
- 15.McQuistion K, Zens T, Jung HS, et al. Insurance status and race affect treatment and outcome of traumatic brain injury. J Surg Res. 2016;205:261–71. [DOI] [PubMed] [Google Scholar]
- 16.Budnick HC, Tyroch AH, Milan SA. Ethnic disparities in traumatic brain injury care referral in a Hispanic-majority population. J Surg Res. 2017;215:231–8. [DOI] [PubMed] [Google Scholar]
- 17.Schiraldi M, Patil CG, Mukherjee D, et al. Effect of insurance and racial disparities on outcomes in traumatic brain injury. Journal of neurological surgery Part A, Cen Eur Neurosurg. 2015;76:224–32. [DOI] [PubMed] [Google Scholar]
- 18.Churilla TM, Egleston B, Bleicher R, et al. Disparities in the Local Management of Breast Cancer in the US according to Health Insurance Status. Breast J. 2017;23:169–76. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen BC, Alawadi ZM, Roife D, et al. Do Socioeconomic Factors and Race Determine the Likelihood of Breast-Conserving Surgery? Clin Breast cancer. 2016;16:93–7. [DOI] [PubMed] [Google Scholar]
- 20.Akinyemiju T, Moore JX, Ojesina AI, et al. Racial disparities in individual breast cancer outcomes by hormone-receptor subtype, area-level socio-economic status and healthcare resources. Breast Cancer Res Treat. 2016;157:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukavsky R, Sariego J. Insurance status effects on stage of diagnosis and surgical options used in the treatment of breast cancer. South Med J. 2015;108:258–61. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien B, Koru-Sengul T, Miao F, et al. Disparities in Overall Survival for Male Breast Cancer Patients in the State of Florida (1996–2007). Clin Breast Cancer. 2015;15:177–87. [DOI] [PubMed] [Google Scholar]
- 23.Yang RL, Newman AS, Lin IC, et al. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013;119:2462–8. [DOI] [PubMed] [Google Scholar]
- 24.Pulte D, Jansen L, Brenner H. Population-Level Differences in Rectal Cancer Survival in Uninsured Patients Are Partially Explained by Differences in Treatment. Oncologist. 2017;22:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu JC, Neugut AI, Wang S, et al. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116:1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Zhang Y, Wei X, et al. Racial disparities in pancreatic neuroendocrine tumors survival: a SEER study. Cancer Med. 2017;6:2745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivero M, Nader ND, Blochle R, et al. Poorer limb salvage in African American men with chronic limb ischemia is due to advanced clinical stage and higher anatomic complexity at presentation. J Vasc Surg. 2016;63:1318–24. [DOI] [PubMed] [Google Scholar]
- 28.Loehrer AP, Hawkins AT, Auchincloss HG, et al. Impact of Expanded Insurance Coverage on Racial Disparities in Vascular Disease: Insights From Massachusetts. Ann Surg. 2016;263:705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien-Irr MS, Harris LM, Dosluoglu HH, Dryjski ML. Procedural trends in the treatment of peripheral arterial disease by insurer status in New York State. J Am Coll Surg. 2012;215:311–21. [DOI] [PubMed] [Google Scholar]
- 30.Durham CA, Mohr MC, Parker FM, et al. The impact of socioeconomic factors on outcome and hospital costs associated with femoropopliteal revascularization. J Vasc Surg. 2010;52:600–6. [DOI] [PubMed] [Google Scholar]
- 31.Kim LK, Swaminathan RV, Minutello RM, et al. Trends in hospital treatments for peripheral arterial disease in the United States and association between payer status and quality of care/outcomes, 2007–2011. Catheter Cardiovasc Interv. 2015;86:864–72. [DOI] [PubMed] [Google Scholar]
- 32.Ladd MR, Pajewski NM, Becher RD, et al. Delays in treatment of pediatric appendicitis: a more accurate variable for measuring pediatric healthcare inequalities? Am Surgeon. 2013;79:875–81. [PubMed] [Google Scholar]
- 33.Lavernia CJ, Villa JM. Does Race Affect Outcomes in Total Joint Arthroplasty? Clin Orthop Relat Res. 2015;473:3535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alas AN, Dunivan GC, Wieslander CK, et al. Health Care Disparities Among English-Speaking and Spanish-Speaking Women With Pelvic Organ Prolapse at Public and Private Hospitals: What Are the Barriers? Female Pelvic Med Reconstr Surg. 2016;22:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riall TS, Townsend CM Jr., Kuo YF, et al. Dissecting racial disparities in the treatment of patients with locoregional pancreatic cancer: a 2-step process. Cancer. 2010;116:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiley S, Meinzen-Derr J. Access to cochlear implant candidacy evaluations: who is not making it to the team evaluations? Int J Audiol. 2009;48:74–9. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Jarl J, Gerdtham UG. Are There Inequities in Treatment of End-Stage Renal Disease in Sweden? A Longitudinal Register-Based Study on Socioeconomic Status-Related Access to Kidney Transplantation. Int J Environ Res Pub Health. 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease C, Prevention. Racial disparities in total knee replacement among Medicare enrollees--United States, 2000–2006. MMWR Morb Mortal Wkly Rep. 2009;58:133–8. [PubMed] [Google Scholar]
- 39.Dunlop DD, Manheim LM, Song J, et al. Age and racial/ethnic disparities in arthritis-related hip and knee surgeries. Med Care. 2008;46:200–8. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CT, Fisher E, Welch HG. Racial disparities in abdominal aortic aneurysm repair among male Medicare beneficiaries. Arch Surg. 2008;143:506–10. [DOI] [PubMed] [Google Scholar]
- 41.Halm EA, Tuhrim S, Wang JJ, et al. Racial and ethnic disparities in outcomes and appropriateness of carotid endarterectomy: impact of patient and provider factors. Stroke. 2009;40:2493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curry WT Jr., Carter BS, Barker FG 2nd. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988–2004. Neurosurgery. 2010;66:427–37. [DOI] [PubMed] [Google Scholar]
- 43.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–97. [DOI] [PubMed] [Google Scholar]
- 44.Mosunjac M, Park J, Strauss A, et al. Time to treatment for patients receiving BCS in a public and a private university hospital in Atlanta. Breast. 2012;18:163–7. [DOI] [PubMed] [Google Scholar]
- 45.Bustami RT, Shulkin DB, O’Donnell N, Whitman ED. Variations in time to receiving first surgical treatment for breast cancer as a function of racial/ethnic background: a cohort study. JRSM Open. 2014;5:2042533313515863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Haberland C, Thurm C, et al. Health outcomes in US children with abdominal pain at major emergency departments associated with race and socioeconomic status. PLoS One. 2015;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise ES, Ladner TR, Song J, et al. Race as a predictor of delay from diagnosis to endarterectomy in clinically significant carotid stenosis. J Vasc Surg. 2015;62:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauder AR, Gross CP, Killelea BK, et al. The Relationship Between Geographic Access to Plastic Surgeons and Breast Reconstruction Rates Among Women Undergoing Mastectomy for Cancer. Ann Plast Surg. 2017;78:324–9. [DOI] [PubMed] [Google Scholar]
- 49.Sharma K, Grant D, Parikh R, Myckatyn T. Race and Breast Cancer Reconstruction: Is There a Health Care Disparity? Plast Reconstr Surg. 2016;138:354–61. [DOI] [PubMed] [Google Scholar]
- 50.Mahmoudi E, Giladi AM, Wu L, Chung KC. Effect of federal and state policy changes on racial/ethnic variation in immediate postmastectomy breast reconstruction. Plast Reconstr Surg. 2015;135:1285–94. [DOI] [PubMed] [Google Scholar]
- 51.Greenberg CC, Schneider EC, Lipsitz SR, et al. Do variations in provider discussions explain socioeconomic disparities in postmastectomy breast reconstruction? J Am Coll Surg. 2008;206:605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kruper L, Holt A, Xu XX, et al. Disparities in reconstruction rates after mastectomy: patterns of care and factors associated with the use of breast reconstruction in Southern California. Ann Surg Oncol. 2011;18:2158–65. [DOI] [PubMed] [Google Scholar]
- 53.Kruper L, Xu X, Henderson K, Bernstein L. Disparities in reconstruction rates after mastectomy for ductal carcinoma in situ (DCIS): patterns of care and factors associated with the use of breast reconstruction for DCIS compared with invasive cancer. Ann Surg Oncol. 2011;18:3210–9. [DOI] [PubMed] [Google Scholar]
- 54.Sisco M, Du H, Warner JP, Howard MA, Winchester DP, Yao K. Have we expanded the equitable delivery of postmastectomy breast reconstruction in the new millennium? Evidence from the national cancer data base. J Am Coll Surg. 2012;215:658–66. [DOI] [PubMed] [Google Scholar]
- 55.Bradley CJ, Dahman B, Shickle LM, Lee W. Surgery wait times and specialty services for insured and uninsured breast cancer patients: does hospital safety net status matter? Health Serv Res. 2012;47:677–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng WH, Stevenson TR, Canter RJ, et al. Sacramento area breast cancer epidemiology study: use of postmastectomy breast reconstruction along the rural-to-urban continuum. Plast Reconstr Surg. 2010;126:1815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubecz A, Solymosi N, Schweigert M, et al. Time trends and disparities in lymphadenectomy for gastrointestinal cancer in the United States: a population-based analysis of 326,243 patients. J Gastrointest Surg. 2013;17:611–8. [DOI] [PubMed] [Google Scholar]
- 59.Taioli E, Flores R. Appropriateness of Surgical Approach in Black Patients with Lung Cancer-15 Years Later, Little Has Changed. J Thorac Oncol. 2017;12:573–7. [DOI] [PubMed] [Google Scholar]
- 60.Loehrer AP, Song Z, Auchincloss HG, Hutter MM. Influence of Health Insurance Expansion on Disparities in the Treatment of Acute Cholecystitis. Ann Surg. 2015;262:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber DJ, Shoham DA, Luke A, et al. Racial odds for amputation ratio in traumatic lower extremity fractures. J Trauma. 2011;71:1732–6. [DOI] [PubMed] [Google Scholar]
- 62.Ricciardi R, Selker HP, Baxter NN, et al. Disparate use of minimally invasive surgery in benign surgical conditions. Surg Endosc. 2008;22:1977–86. [DOI] [PubMed] [Google Scholar]
- 63.Mehta A, Xu T, Hutfless S, et al. Patient, surgeon, and hospital disparities associated with benign hysterectomy approach and perioperative complications. Am J Obstet Gynecol. 2017;216:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abenhaim HA, Azziz R, Hu J, et al. Socioeconomic and racial predictors of undergoing laparoscopic hysterectomy for selected benign diseases: analysis of 341487 hysterectomies. J Minim Invasive Gynecol. 2008;15:11–5. [DOI] [PubMed] [Google Scholar]
- 65.Loehrer AP, Song Z, Auchincloss HG, Hutter MM. Massachusetts health care reform and reduced racial disparities in minimally invasive surgery. JAMA Surg. 2013;148:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olaya W, Wong JH, Morgan JW, et al. Disparities in the surgical management of women with stage I breast cancer. Am Surg. 2009;75:869–72. [PubMed] [Google Scholar]
- 67.Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, et al. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer epidemiol. 2015;39:745–51. [DOI] [PubMed] [Google Scholar]
- 68.Alderman AK, Bynum J, Sutherland J, et al. Surgical treatment of breast cancer among the elderly in the United States. Cancer. 2011;117:698–704. [DOI] [PubMed] [Google Scholar]
- 69.Zafar SN, Changoor NR, Williams K, et al. Race and socioeconomic disparities in national stoma reversal rates. Am J Surg. 2016;211:710–5. [DOI] [PubMed] [Google Scholar]
- 70.Dodwell E, Wright J, Widmann R, et al. Socioeconomic Factors Are Associated With Trends in Treatment of Pediatric Femoral Shaft Fractures, and Subsequent Implant Removal in New York State. J Pediatr Orthop. 2016;36:459–64. [DOI] [PubMed] [Google Scholar]
- 71.Campbell JE, Janitz AE, Vesely SK, et al. Patterns of Care for Localized Breast Cancer in Oklahoma, 2003–2006. Women Health. 2015;55:975–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Epstein AJ, Gray BH, Schlesinger M. Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg. 2010;145:179–86. [DOI] [PubMed] [Google Scholar]
- 73.Popescu I, Schrag D, Ang A, Wong M. Racial/Ethnic and Socioeconomic Differences in Colorectal and Breast Cancer Treatment Quality: The Role of Physician-level Variations in Care. Med Care. 2016;54:780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Esnaola NF, Knott K, Finney C, et al. Urban/rural residence moderates effect of race on receipt of surgery in patients with nonmetastatic breast cancer: a report from the South Carolina central cancer registry. Ann Surg Oncol. 2008;15:1828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keating NL, Kouri E, He Y, et al. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Med Care. 2009;47:765–73. [DOI] [PubMed] [Google Scholar]
- 76.Black DM, Jiang J, Kuerer HM, et al. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149:788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosson GD, Singh NK, Ahuja N, et al. Multilevel analysis of the impact of community vs patient factors on access to immediate breast reconstruction following mastectomy in Maryland. Arch Surg. 2008;143:1076–81. [DOI] [PubMed] [Google Scholar]
- 78.Offodile AC 2nd, Tsai TC, Wenger JB, Guo L. Racial disparities in the type of postmastectomy reconstruction chosen. J Surg Res. 2015;195:368–76. [DOI] [PubMed] [Google Scholar]
- 79.Amaranto DJ, Abbas F, Krantz S, et al. An evaluation of gender and racial disparity in the decision to treat surgically arterial disease. J Vasc Surg. 2009;50:1340–7. [DOI] [PubMed] [Google Scholar]
- 80.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335:791–9. [DOI] [PubMed] [Google Scholar]
- 81.Katz JN. Patient preferences and health disparities. JAMA. 2001;286:1506–9. [DOI] [PubMed] [Google Scholar]
- 82.Voorhees K, Fernald DH, Emsermann C, et al. Underinsurance in primary care: a report from the State Networks of Colorado Ambulatory Practices and Partners (SNOCAP). J Am Board Fam Med. 2008;21:309–16. [DOI] [PubMed] [Google Scholar]
- 83.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–9. [DOI] [PubMed] [Google Scholar]
- 84.Haider AH, Schneider EB, Sriram N, et al. Unconscious race and social class bias among acute care surgical clinicians and clinical treatment decisions. JAMA Surg. 2015;150:457–64. [DOI] [PubMed] [Google Scholar]
- 85.Haider AH, Sexton J, Sriram N, et al. Association of unconscious race and social class bias with vignette-based clinical assessments by medical students. JAMA. 2011;306:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dovidio JF, Gaertner SL. Aversive racism and selection decisions: 1989 and 1999. Psychol Sci. 2000;11:315–9. [DOI] [PubMed] [Google Scholar]
- 87.Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22:1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care. 2008;46:678–85. [DOI] [PubMed] [Google Scholar]
- 89.Tamayo-Sarver JH, Dawson NV, Hinze SW, et al. The effect of race/ethnicity and desirable social characteristics on physicians’ decisions to prescribe opioid analgesics. Acad Emerg Med. 2003;10:1239–48. [DOI] [PubMed] [Google Scholar]
- 90.Loehrer AP, Chang DC, Hutter MM, et al. Health Insurance Expansion and Treatment of Pancreatic Cancer: Does Increased Access Lead to Improved Care? J Am Coll Surg. 2015;221:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patzer RE, Plantinga LC, Paul S, et al. Variation in Dialysis Facility Referral for Kidney Transplantation Among Patients With End-Stage Renal Disease in Georgia. JAMA. 2015;314:582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Qurayshi Z, Randolph GW, Srivastav S, Kandil E. Outcomes in endocrine cancer surgery are affected by racial, economic, and healthcare system demographics. Laryngoscope. 2016;126:775–81. [DOI] [PubMed] [Google Scholar]
- 93.Hoehn RS, Hanseman DJ, Dhar VK, et al. Opportunities to Improve Care of Hepatocellular Carcinoma in Vulnerable Patient Populations. J Am Coll Surg. 2017;224:697–704. [DOI] [PubMed] [Google Scholar]
- 94.Chang DC, Zhang Y, Mukherjee D, et al. Variations in referral patterns to high-volume centers for pancreatic cancer. J Am Coll Surg. 2009;209:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang W, Lyman S, Boutin-Foster C, et al. Racial and Ethnic Disparities in Utilization Rate, Hospital Volume, and Perioperative Outcomes After Total Knee Arthroplasty. J Bone Joint Surg Am. 2016;98:1243–52. [DOI] [PubMed] [Google Scholar]
- 96.SooHoo NF, Farng E, Zingmond DS. Disparities in the utilization of high-volume hospitals for total hip replacement. J Natl Med Assoc. 2011;103:31–5. [DOI] [PubMed] [Google Scholar]
- 97.Al-Qurayshi Z, Randolph GW, Srivastav S, et al. Outcomes in thyroid surgery are affected by racial, economic, and healthcare system demographics. Laryngoscope. 2016;126:2194–9. [DOI] [PubMed] [Google Scholar]
- 98.Hauch A, Al-Qurayshi Z, Friedlander P, Kandil E. Association of socioeconomic status, race, and ethnicity with outcomes of patients undergoing thyroid surgery. JAMA Otolaryngol Head Neck Surg. 2014;140:1173–83. [DOI] [PubMed] [Google Scholar]
- 99.Mehta RH, Shahian DM, Sheng S, et al. Association of Hospital and Physician Characteristics and Care Processes With Racial Disparities in Procedural Outcomes Among Contemporary Patients Undergoing Coronary Artery Bypass Grafting Surgery. Circulation. 2016;133:124–30. [DOI] [PubMed] [Google Scholar]
- 100.Bao Y, Kamble S. Geographical distribution of surgical capabilities and disparities in the use of high-volume providers: the case of coronary artery bypass graft. Med Care. 2009;47:794–802. [DOI] [PubMed] [Google Scholar]
- 101.Hauch A, Al-Qurayshi Z, Kandil E. The effect of race and socioeconomic status on outcomes following adrenal operations. J Surg Onc. 2015;112:822–7. [DOI] [PubMed] [Google Scholar]
- 102.Kim J, ElRayes W, Wilson F, et al. Disparities in the receipt of robot-assisted radical prostatectomy: between-hospital and within-hospital analysis using 2009–2011 California inpatient data. BMJ open. 2015;5:007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pollack CE, Bekelman JE, Epstein AJ, et al. Racial disparities in changing to a high-volume urologist among men with localized prostate cancer. Med Care. 2011;49:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang LC, Tran TB, Ma Y, et al. Factors that influence minority use of high-volume hospitals for colorectal cancer care. Dis Colon Rectum. 2015;58:526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hicks CW, Hashmi ZG, Hui X, et al. Explaining the Paradoxical Age-based Racial Disparities in Survival After Trauma: The Role of the Treating Facility. Ann Surg. 2015;262:179–83. [DOI] [PubMed] [Google Scholar]
- 106.Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011;53:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mukherjee D, Kosztowski T, Zaidi HA, et al. Disparities in access to pediatric neurooncological surgery in the United States. Pediatrics. 2009;124:688–96. [DOI] [PubMed] [Google Scholar]
- 108.Mukherjee D, Zaidi HA, Kosztowski TA, et al. Variations in referral patterns for hypophysectomies among pediatric patients with sellar and parasellar tumors. Childs Nerv Syst. 2010;26:305–11. [DOI] [PubMed] [Google Scholar]
- 109.Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Onc. 2010;17:2824–31. [DOI] [PubMed] [Google Scholar]
- 110.Bristow RE, Zahurak ML, Ibeanu OA. Racial disparities in ovarian cancer surgical care: a population-based analysis. Gynecol Oncol. 2011;121:364–8. [DOI] [PubMed] [Google Scholar]
- 111.Liu FW, Randall LM, Tewari KS, Bristow RE. Racial disparities and patterns of ovarian cancer surgical care in California. Gynecol Oncol. 2014;132:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Armstrong K, Randall TC, Polsky D, et al. Racial differences in surgeons and hospitals for endometrial cancer treatment. Med Care. 2011;49:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fleury AC, Ibeanu OA, Bristow RE. Racial disparities in surgical care for uterine cancer. Gynecol Onc. 2011;121:571–6. [DOI] [PubMed] [Google Scholar]
- 114.Schroeck FR, Hollingsworth JM, Hollenbeck BK, et al. Differential adoption of laser prostatectomy for treatment of benign prostatic hyperplasia. Urology. 2013;81:1177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nuno M, Drazin DG, Acosta FL Jr.. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13:116–23. [DOI] [PubMed] [Google Scholar]
- 116.Sun M, Karakiewicz PI, Sammon JD, et al. Disparities in selective referral for cancer surgeries: implications for the current healthcare delivery system. BMJ open. 2014;4:003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Attenello FJ, Adamczyk P, Wen G, et al. Racial and socioeconomic disparities in access to mechanical revascularization procedures for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jacobs AJ, Lindholm EB, Levy CF, et al. Racial and ethnic disparities in treatment and survival of pediatric sarcoma. The J Surg Res. 2017;219:43–9. [DOI] [PubMed] [Google Scholar]
- 119.Garner EF, Maizlin II, Dellinger MB, et al. Effects of socioeconomic status on children with well-differentiated thyroid cancer. Surgery. 2017;162:662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamilton EC, Nguyen HT, Chang YC, et al. Health Disparities Influence Childhood Melanoma Stage at Diagnosis and Outcome. J Pediatr. 2016;175:182–7. [DOI] [PubMed] [Google Scholar]
- 121.Austin MT, Hamilton E, Zebda D, et al. Health disparities and impact on outcomes in children with primary central nervous system solid tumors. J Neurosurg Pediatr. 2016;18:585–93. [DOI] [PubMed] [Google Scholar]
- 122.Shapiro M, Chen Q, Huang Q, et al. Associations of Socioeconomic Variables With Resection, Stage, and Survival in Patients With Early-Stage Pancreatic Cancer. JAMA Surg. 2016;151:338–45. [DOI] [PubMed] [Google Scholar]
- 123.Bregar AJ, Alejandro Rauh-Hain J, Spencer R, et al. Disparities in receipt of care for high-grade endometrial cancer: A National Cancer Data Base analysis. Gynecol Oncol. 2017;145:114–21. [DOI] [PubMed] [Google Scholar]
- 124.Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC. Disparities in treatment and survival for women with endometrial cancer: A contemporary national cancer database registry analysis. Gynecol Oncol. 2016;143:98–104. [DOI] [PubMed] [Google Scholar]
- 125.Churilla T, Egleston B, Dong Y, et al. Disparities in the management and outcome of cervical cancer in the United States according to health insurance status. Gynecol Oncol. 2016;141:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eng TY, Chen T, Vincent J, et al. Persistent Disparities in Hispanics with Cervical Cancer in a Major City. J Racial Ethn Health Disparities. 2017;4:165–8. [DOI] [PubMed] [Google Scholar]
- 127.Fleming S, Schluterman NH, Tracy JK, Temkin SM. Black and white women in Maryland receive different treatment for cervical cancer. PLoS One. 2014;9:104344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abdelsattar ZM, Hendren S, Wong SL. The impact of health insurance on cancer care in disadvantaged communities. Cancer. 2017;123:1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uhr JH, Shah J, Warner RR, Divino CM. Racial Disparities in Clinical Presentation and Surgical Outcomes in Patients with Small Bowel Carcinoid Tumors. Am Surg. 2016;82:89–92. [PubMed] [Google Scholar]
- 130.Alamanda VK, Song Y, Schwartz HS, Holt GE. Racial Disparities in Extremity Soft-Tissue Sarcoma Outcomes: A Nationwide Analysis. Am J Clin Oncol. 2015;38:595–9. [DOI] [PubMed] [Google Scholar]
- 131.Zagzag J, Kenigsberg A, Patel KN, Heller KS, Ogilvie JB. Thyroid cancer is more likely to be detected incidentally on imaging in private hospital patients. J Surg Res. 2017;215:239–44. [DOI] [PubMed] [Google Scholar]
- 132.Babu R, Sharma R, Bagley JH, et al. Vestibular schwannomas in the modern era: epidemiology, treatment trends, and disparities in management. J Neurosurg. 2013;119:121–30. [DOI] [PubMed] [Google Scholar]
- 133.Ellis SD, Blackard B, Carpenter WR, et al. Receipt of National Comprehensive Cancer Network guideline-concordant prostate cancer care among African American and Caucasian American men in North Carolina. Cancer. 2013;119:2282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dua A, Desai SS, Heller JA. The Impact of Race on Advanced Chronic Venous Insufficiency. Ann Vasc Surg. 2016;34:152–6. [DOI] [PubMed] [Google Scholar]
- 135.Dua A, Heller JA. Advanced Chronic Venous Insufficiency. Vascular and endovascular surgery. 2017;51:12–6. [DOI] [PubMed] [Google Scholar]
- 136.Selvarajah S, Black JH 3rd, Haider AH, Abularrage CJ. Racial disparity in early graft failure after infrainguinal bypass. Journal Surg Res. 2014;190:335–43. [DOI] [PubMed] [Google Scholar]
- 137.Hughes K, Seetahal S, Oyetunji T, et al. Racial/ethnic disparities in amputation and revascularization: a nationwide inpatient sample study. Vasc Endovascular Surg. 2014;48:34–7. [DOI] [PubMed] [Google Scholar]
- 138.Durazzo TS, Frencher S, Gusberg R. Influence of race on the management of lower extremity ischemia: revascularization vs amputation. JAMA Surg. 2013;148:617–23. [DOI] [PubMed] [Google Scholar]
- 139.Brinjikji W, El-Sayed AM, Kallmes DF, et al. Racial and insurance based disparities in the treatment of carotid artery stenosis: a study of the Nationwide Inpatient Sample. J Neurointerv Surg. 2015;7:695–702. [DOI] [PubMed] [Google Scholar]
- 140.Brown ZD, Bey AK, Bonfield CM, et al. Racial disparities in health care access among pediatric patients with craniosynostosis. J Neurosurg Pediatr. 2016;18:269–74. [DOI] [PubMed] [Google Scholar]
- 141.Lin Y, Pan IW, Harris DA, et al. The Impact of Insurance, Race, and Ethnicity on Age at Surgical Intervention among Children with Nonsyndromic Craniosynostosis. J Pediatr. 2015;166:1289–96. [DOI] [PubMed] [Google Scholar]
- 142.Boomer L, Freeman J, Landrito E, Feliz A. Perforation in adults with acute appendicitis linked to insurance status, not ethnicity. J Surg Res. 2010;163:221–4. [DOI] [PubMed] [Google Scholar]
- 143.Calfee RP, Shah CM, Canham CD, et al. The influence of insurance status on access to and utilization of a tertiary hand surgery referral center. J Bone Joint Surg Am. 2012;94:2177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hausmann LR, Mor M, Hanusa BH, et al. The effect of patient race on total joint replacement recommendations and utilization in the orthopedic setting. J Gen Intern Med. 2010;25:982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chidi AP, Bryce CL, Myaskovsky L, et al. Differences in Physician Referral Drive Disparities in Surgical Intervention for Hepatocellular Carcinoma: A Retrospective Cohort Study. Ann Surg. 2016;263:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979–2003. Cancer. 2011;117:3242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suneja G, Lin CC, Simard EP, et al. Disparities in cancer treatment among patients infected with the human immunodeficiency virus. Cancer. 2016;122:2399–407. [DOI] [PubMed] [Google Scholar]
- 148.Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection: a key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer. 2009;115:3979–90. [DOI] [PubMed] [Google Scholar]
- 149.Cheung MC, Yang R, Byrne MM, et al. Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? Cancer. 2010;116:723–33. [DOI] [PubMed] [Google Scholar]
- 150.Rauh-Hain JA, Buskwofie A, Clemmer J, et al. Racial disparities in treatment of high-grade endometrial cancer in the Medicare population. Obstet Gynecol. 2015;125:843–51. [DOI] [PubMed] [Google Scholar]
- 151.Gray PJ, Lin CC, Cooperberg MR, et al. Temporal Trends and the Impact of Race, Insurance, and Socioeconomic Status in the Management of Localized Prostate Cancer. Eur Urol. 2017;71:729–37. [DOI] [PubMed] [Google Scholar]
- 152.Yang R, Cheung MC, Byrne MM, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010;116:2437–47. [DOI] [PubMed] [Google Scholar]
- 153.Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suga JM, Nguyen DV, Mohammed SM, et al. Racial disparities on the use of invasive and noninvasive staging in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5:1772–8. [DOI] [PubMed] [Google Scholar]
- 155.Shugarman LR, Mack K, Sorbero ME, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–81. [DOI] [PubMed] [Google Scholar]
- 156.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yorio JT, Yan J, Xie Y, Gerber DE. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clin Lung Cancer. 2012;13:448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oliver JB, Son JY, Bongu A, et al. Colorectal Cancer Disparities at an Urban Tertiary Care Center. Am Surg. 2016;82:181–3. [PubMed] [Google Scholar]
- 159.Neuman HB, O’Connor ES, Weiss J, et al. Surgical treatment of colon cancer in patients aged 80 years and older : analysis of 31,574 patients in the SEER-Medicare database. Cancer. 2013;119:639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Long B, Chang J, Ziogas A, Tewari KS, et al. Impact of race, socioeconomic status, and the health care system on the treatment of advanced-stage ovarian cancer in California. Am J Obstet Gynecol. 2015;212:468. [DOI] [PubMed] [Google Scholar]
- 161.Fairfield KM, Lucas FL, Earle CC, et al. Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the Medicare population. Cancer. 2010;116:4840–8. [DOI] [PubMed] [Google Scholar]
- 162.Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg. 2011;146:778–84. [DOI] [PubMed] [Google Scholar]
- 163.Revels SL, Morris AM, Reddy RM, et al. Racial disparities in esophageal cancer outcomes. Ann Surg Oncol. 2013;20:1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Missios S, Bekelis K. The association of insurance status and race with the procedural volume of traumatic brain injury patients. Injury. 2016;47:154–9. [DOI] [PubMed] [Google Scholar]
- 165.Woo K, Gascue L, Goldman DP, Romley JA. Variations in outcomes of hemodialysis vascular access by race/ethnicity in the elderly. J Vasc Surg. 2017;65:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hawkins K, Escoto KH, Ozminkowski RJ, et al. Disparities in major joint replacement surgery among adults with Medicare supplement insurance. Popul Health Manag. 2011;14:231–8. [DOI] [PubMed] [Google Scholar]
- 167.Wetterholm M, Turkiewicz A, Stigmar K, et al. The rate of joint replacement in osteoarthritis depends on the patient’s socioeconomic status. Acta Orthop. 2016;87:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Patel N, Deshmukh A, Thakkar B, et al. Gender, Race, and Health Insurance Status in Patients Undergoing Catheter Ablation for Atrial Fibrillation. Am J Cardiol. 2016;117:1117–26. [DOI] [PubMed] [Google Scholar]
- 169.Rodriguez F, Foody JM, Wang Y, Lopez L. Young Hispanic Women Experience Higher In-Hospital Mortality Following an Acute Myocardial Infarction. J Am Heart Assoc. 2015;4:002089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Albert MA, Ayanian JZ, Silbaugh TS, et al. Early results of Massachusetts healthcare reform on racial, ethnic, and socioeconomic disparities in cardiovascular care. Circulation. 2014;129:2528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yeung M, Kerrigan J, Sodhi S, et al. Racial differences in rates of aortic valve replacement in patients with severe aortic stenosis. Am J Cardiol. 2013;112:991–5. [DOI] [PubMed] [Google Scholar]
- 172.Bhogal SK, Reddigan JI, Rotstein OD, et al. Inequity to the utilization of bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2015;25:888–99. [DOI] [PubMed] [Google Scholar]
- 173.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6:8–15. [DOI] [PubMed] [Google Scholar]
- 174.Birkmeyer NJ, Gu N. Race, socioeconomic status, and the use of bariatric surgery in Michigan. Obes Surg. 2012;22:259–65. [DOI] [PubMed] [Google Scholar]
- 175.Worni M, Guller U, Maciejewski ML, et al. Racial differences among patients undergoing laparoscopic gastric bypass surgery: a population-based trend analysis from 2002 to 2008. Obes Surg. 2013;23:226–33. [DOI] [PubMed] [Google Scholar]
- 176.Smith DF, Boss EF. Racial/ethnic and socioeconomic disparities in the prevalence and treatment of otitis media in children in the United States. Laryngoscope. 2010;120:2306–12. [DOI] [PubMed] [Google Scholar]
- 177.Willis AW, Schootman M, Kung N, et al. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. 2014;82:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Frey MK, Moss HA, Musa F, et al. Preoperative experience for public hospital patients with gynecologic cancer: Do structural barriers widen the gap? Cancer. 2016;122:859–67. [DOI] [PubMed] [Google Scholar]
- 179.Routh JC, Pennison M, Rosoklija I, et al. Racial variation in timing of pyeloplasty: prenatal versus postnatal diagnosis. J Urol. 2011;186:2386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boss EF, Benke JR, Tunkel DE, et al. Public insurance and timing of polysomnography and surgical care for children with sleep-disordered breathing. JAMA Otolaryngol Head Neck Surg. 2015;141:106–11. [DOI] [PubMed] [Google Scholar]
- 181.Feinglass J, Abadin S, Thompson J, Pearce WH. A census-based analysis of racial disparities in lower extremity amputation rates in Northern Illinois, 1987–2004. J Vasc Surg. 2008;47:1001–7. [DOI] [PubMed] [Google Scholar]
- 182.Murphy EH, Davis CM, Modrall JG, et al. Effects of ethnicity and insurance status on outcomes after thoracic endoluminal aortic aneurysm repair (TEVAR). J Vasc Surg. 2010;51:14–20. [DOI] [PubMed] [Google Scholar]
- 183.Shah AD, Kohli N, Rajan SS, Hoyte L. Surgery for stress urinary incontinence in the United States: does race play a role? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1085–92. [DOI] [PubMed] [Google Scholar]
- 184.Hayanga AJ, Kaiser HE, Sinha R, et al. Residential segregation and access to surgical care by minority populations in US counties. J Am Coll Surg. 2009;208:1017–22. [DOI] [PubMed] [Google Scholar]
- 185.Francis ML, Scaife SL, Zahnd WE, et al. Joint replacement surgeries among medicare beneficiaries in rural compared with urban areas. Arthritis Rheum. 2009;60:3554–62. [DOI] [PubMed] [Google Scholar]
- 186.Lee SL, Yaghoubian A, Stark R, Shekherdimian S. Equal access to healthcare does not eliminate disparities in the management of adults with appendicitis. J Surg Res. 2011;170:209–13. [DOI] [PubMed] [Google Scholar]
- 187.Mountford WK, Lackland DT, Soule JB, et al. Racial disparities in trends for cardiovascular disease and procedures among hospitalized diabetic patients. Ethn Dis. 2008;18:131–5. [PubMed] [Google Scholar]
- 188.Rose KM, Foraker RE, Heiss G, et al. Neighborhood socioeconomic and racial disparities in angiography and coronary revascularization: the ARIC surveillance study. Ann Epidemiol. 2012;22:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Skolasky RL, Maggard AM, Thorpe RJ Jr., et al. United States hospital admissions for lumbar spinal stenosis: racial and ethnic differences, 2000 through 2009. Spine. 2013;38:2272–8. [DOI] [PubMed] [Google Scholar]
- 190.Andrews A, Franklin L, Rush N, et al. Age, Sex, Health Insurance, and Race Associated With Increased Rate of Emergent Pediatric Gastrointestinal Procedures. J Pediatr Gastroenterol Nutr. 2017;64:907–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Arnaoutakis DJ, Propper BW, Black JH 3rd, et al. Racial and ethnic disparities in the treatment of unruptured thoracoabdominal aortic aneurysms in the United States. Journal Surg Res. 2013;184:651–7. [DOI] [PubMed] [Google Scholar]
- 192.Schwartz DA, Hui X, Schneider EB, et al. Worse outcomes among uninsured general surgery patients: does the need for an emergency operation explain these disparities? Surgery. 2014;156:345–51. [DOI] [PubMed] [Google Scholar]
- 193.Peters NA, Javed AA, He J, et al. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J Surg Res. 2017;210:253–60. [DOI] [PubMed] [Google Scholar]
- 194.Sarpel U, Suprun M, Sofianou A, et al. Disentangling the effects of race and socioeconomic factors on liver transplantation rates for hepatocellular carcinoma. Clin Transplant. 2016;30:714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Robbins AS, Cox DD, Johnson LB, Ward EM. Persistent disparities in liver transplantation for patients with hepatocellular carcinoma in the United States, 1998 through 2007. Cancer. 2011;117:4531–9. [DOI] [PubMed] [Google Scholar]
- 196.Wilder JM, Oloruntoba OO, Muir AJ, Moylan CA. Role of patient factors, preferences, and distrust in health care and access to liver transplantation and organ donation. Liver Transpl. 2016;22:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Moylan CA, Brady CW, Johnson JL, et al. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kemmer N, Alsina A, Neff GW. Orthotopic liver transplantation in a multiethnic population: role of spatial accessibility. Transplant Proc. 2011;43:3780–2. [DOI] [PubMed] [Google Scholar]
- 199.Talamantes E, Norris KC, Mangione CM, et al. Linguistic Isolation and Access to the Active Kidney Transplant Waiting List in the United States. Clin J Am Soc Nephrol. 2017;12:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5:2276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12:358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013. January;95:309–18. [DOI] [PubMed] [Google Scholar]
- 203.Grams ME, Chen BP, Coresh J, Segev DL. Preemptive deceased donor kidney transplantation: considerations of equity and utility. Clin J Am Soc Nephrol. 2013;8:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Johansen KL, Zhang R, Huang Y, et al. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2012;7:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Patzer RE, Sayed BA, Kutner N, et al. Racial and ethnic differences in pediatric access to preemptive kidney transplantation in the United States. Am J Transplant. 2013;13:1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Quon BS, Psoter K, Mayer-Hamblett N, Aitken ML, et al. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. Am J Respir Crit Care Med. 2012;186:1008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Holliday TL, Suggs PD, Thompson SN, Richmond BK. Disparities in Rural Breast Cancer Care: Factors Affecting Choice of Breast Reconstruction in a West Virginia Tertiary Care Hospital. Am Surg. 2017;83:717–21. [PubMed] [Google Scholar]
- 208.Grimmer L, Liederbach E, Velasco J, et al. Variation in Contralateral Prophylactic Mastectomy Rates According to Racial Groups in Young Women with Breast Cancer, 1998 to 2011: A Report from the National Cancer Data Base. J Am Coll Surg. 2015;221:187–96. [DOI] [PubMed] [Google Scholar]
- 209.Salasky V, Yang RL, Datta J, et al. Racial disparities in the use of outpatient mastectomy. J Surg Res. 2014;186:16–22. [DOI] [PubMed] [Google Scholar]
- 210.Magnetta MJ, Xing M, Zhang D, Kim HS. The Effect of Bridging Locoregional Therapy and Sociodemographics on Survival in Hepatocellular Carcinoma Patients Undergoing Orthotopic Liver Transplantation: A United Network for Organ Sharing Population Study. J Vasc Interv Radiol : JVIR. 2016;27:1822–8. [DOI] [PubMed] [Google Scholar]
- 211.Martinez SR, Robbins AS, Meyers FJ, et al. Racial and ethnic differences in treatment and survival among adults with primary extremity soft-tissue sarcoma. Cancer. 2008;112:1162–8. [DOI] [PubMed] [Google Scholar]
- 212.Downing S, Ahuja N, Oyetunji TA, et al. Disparity in limb-salvage surgery among sarcoma patients. Am J Surg. 2010;199:549–53. [DOI] [PubMed] [Google Scholar]
- 213.Parikh-Patel A, Morris CR, Kizer KW. Disparities in quality of cancer care: The role of health insurance and population demographics. Medicine. 2017;96:9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hayn MH, Orom H, Shavers VL, et al. Racial/ethnic differences in receipt of pelvic lymph node dissection among men with localized/regional prostate cancer. Cancer. 2011;117:4651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zarkowsky DS, Arhuidese IJ, Hicks CW, et al. Racial/Ethnic Disparities Associated With Initial Hemodialysis Access. JAMA Surg. 2015;150:529–36. [DOI] [PubMed] [Google Scholar]
- 216.Agarwal S, Menon V, Jaber WA. Outcomes after acute ischemic stroke in the United States: does residential ZIP code matter? J Am Heart Assoc. 2015;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jin J, Sandoval V, Lawless ME, et al. Disparity in the management of Graves’ disease observed at an urban county hospital: a decade-long experience. Am J Surg. 2012;204:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bolorunduro OB, Haider AH, Oyetunji TA, et al. Disparities in trauma care: are fewer diagnostic tests conducted for uninsured patients with pelvic fracture? Am J Surg. 2013;205:365–70. [DOI] [PubMed] [Google Scholar]
- 219.Casale JC, Wolf F, Pei Y, Devereux RB. Socioeconomic and ethnic disparities in the use of biventricular pacemakers in heart failure patients with left ventricular systolic dysfunction. Ethn Dis. 2013;23:275–80. [PubMed] [Google Scholar]
- 220.Menendez ME, Ring D. Minorities are less likely to receive autologous blood transfusion for major elective orthopaedic surgery. Clin Orthop Relat Res. 2014;472:3559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Damle RN, Flahive JM, Davids JS, et al. Examination of Racial Disparities in the Receipt of Minimally Invasive Surgery Among a National Cohort of Adult Patients Undergoing Colorectal Surgery. Dis Colon Rectum. 2016;59:1055–62. [DOI] [PubMed] [Google Scholar]
- 222.Robinson CN, Balentine CJ, Sansgiry S, Berger DH. Disparities in the use of minimally invasive surgery for colorectal disease. J Gastrointest Surg. 2012;16:897–903. [DOI] [PubMed] [Google Scholar]
- 223.Alnasser M, Schneider EB, Gearhart SL, et al. National disparities in laparoscopic colorectal procedures for colon cancer. Surg Endosc. 2014;28:49–57. [DOI] [PubMed] [Google Scholar]
- 224.Greenstein AJ, Romanoff AM, Moskowitz AJ, et al. Payer status and access to laparoscopic subtotal colectomy for ulcerative colitis. Dis Colon Rectum. 2013;56:1062–7. [DOI] [PubMed] [Google Scholar]
- 225.Oyetunji TA, Nwomeh BC, Ong’uti SK, et al. Laparoscopic appendectomy in children with complicated appendicitis: ethnic disparity amid changing trend. J Surg Res. 2011;170:99–103. [DOI] [PubMed] [Google Scholar]
- 226.Vogel TR, Cantor JC, Dombrovskiy VY, et al. AAA repair: sociodemographic disparities in management and outcomes. Vasc Endovascular Surg. 2008;42:555–60. [DOI] [PubMed] [Google Scholar]
- 227.Osborne NH, Mathur AK, Upchurch GR Jr., Dimick JB. Understanding the racial disparity in the receipt of endovascular abdominal aortic aneurysm repair. Arch Surg. 2010;145:1105–8. [DOI] [PubMed] [Google Scholar]
- 228.Johnston WF, LaPar DJ, Newhook TE, et al. Association of race and socioeconomic status with the use of endovascular repair to treat thoracic aortic diseases. J Vasc Surg. 2013;58:1476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rowe VL, Weaver FA, Lane JS, Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998–2006. J Vasc Surg. 2010;51:21–6. [DOI] [PubMed] [Google Scholar]
- 230.Lefebvre KM, Chevan J. Sex disparities in level of amputation. Arch Phys Med Rehabil. 2011;92:118–24. [DOI] [PubMed] [Google Scholar]
- 231.Poulose BK, Phillips S, Nealon W, et al. Choledocholithiasis management in rural America: health disparity or health opportunity? J Surg Res. 2011;170:214–9. [DOI] [PubMed] [Google Scholar]
- 232.Trinh QD, Schmitges J, Sun M, et al. Improvement of racial disparities with respect to the utilization of minimally invasive radical prostatectomy in the United States. Cancer. 2012;118:1894–900. [DOI] [PubMed] [Google Scholar]
- 233.Martinez SR, Tseng WH, Shah DR, et al. Urban and non-urban disparities in the use of post-mastectomy radiation for breast cancer. Med Oncol. 2012;29:1523–8. [DOI] [PubMed] [Google Scholar]
- 234.Metcalfe D, Davis WA, Olufajo OA, et al. Access to post-discharge inpatient care after lower limb trauma. J Surg Res. 2016;203:140–4. [DOI] [PubMed] [Google Scholar]
- 235.Gerry JM, Weiser TG, Spain DA, Staudenmayer KL. Uninsured status may be more predictive of outcomes among the severely injured than minority race. Injury. 2016;47:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association Between Geographic Access to Cancer Care, Insurance, and Receipt of Chemotherapy: Geographic Distribution of Oncologists and Travel Distance. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2015;33:3177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chang DT, Ko AB, Murray GS, et al. Lack of financial barriers to pediatric cochlear implantation: impact of socioeconomic status on access and outcomes. Arch Otolaryngol Head Neck Surg. 2010;136:648–57. [DOI] [PubMed] [Google Scholar]