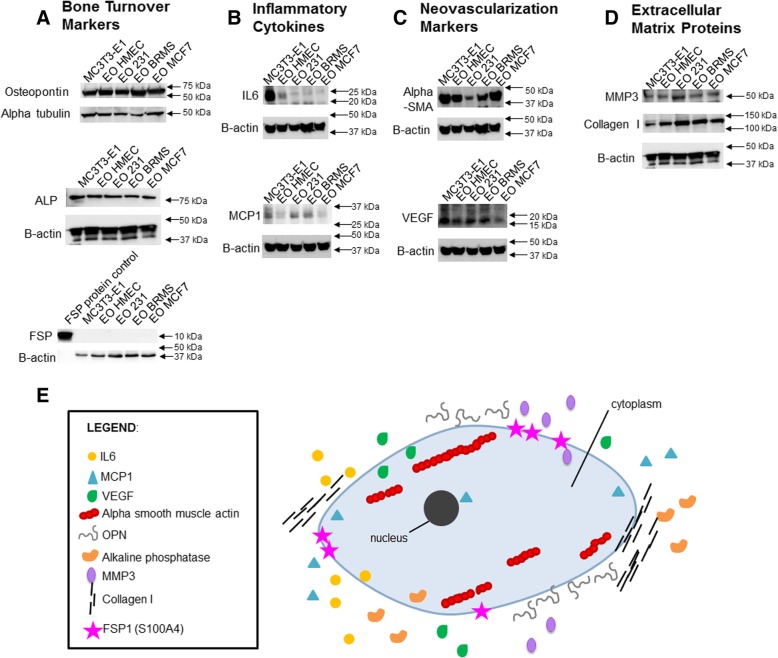
Fig. 2.
EO cells express different markers than normal osteoblasts. MC3T3-E1 cells were plated at 1 × 105 cells/cm2 in 35 × 10 mm dishes. Twenty-four hours later, the media were replaced with differentiation medium, and cells grown to late differentiation (20 days). Differentiation medium was exchanged every third day. EO cells were plated at 1 × 105 cells/cm2 and grown in three parts 1.5× differentiation medium plus one part either MDA-MB-231, MDA-MB-231BRMS, or MCF-7 breast cancer-conditioned medium or hTERT-HME1 mammary epithelial cell-conditioned medium. Media were changed every second day. To collect cell lysates, growth or differentiation media were removed, and cells washed with cold PBS, then lysates removed using ice-cold RIPA buffer. EO and MC3T3-E1 lysates were quantitated, then subjected to western blotting for proteins associated with a bone turnover (osteopontin (OPN), alkaline phosphatase (ALP), and fibroblast-specific protein (FSP)), b inflammatory cytokines (IL-6 and MCP-1), c neovascularization (alpha-smooth muscle actin (alpha-SMA) and VEGF) and d extracellular matrix (MMP3 and type I collagen). EO variants examined include EO cells made with hTERT-HME human mammary epithelial (EO HMEC), MDA-MB-231 human metastatic breast cancer (EO 231), MDA-MB-231BRMS human breast cancer metastasis-suppressed (EO BRMS), or MCF-7 human estrogen receptor-positive breast cancer (EO MCF7)-conditioned medium. Three biological replicates were carried out per condition, per time, and the experiment is repeated twice. Shown are representative results. e A “EO” can be defined as an osteoblast-like cell with altered expression of four defining characteristics which distinguish it from a normal osteoblast: bone turnover markers (OPN, ALP, and FSP), inflammatory cytokines (IL-6 and MCP-1), neovascularization (alpha-SMA and VEGF), and extracellular matrix markers (MMP3 and collagen I)