Figure 1.
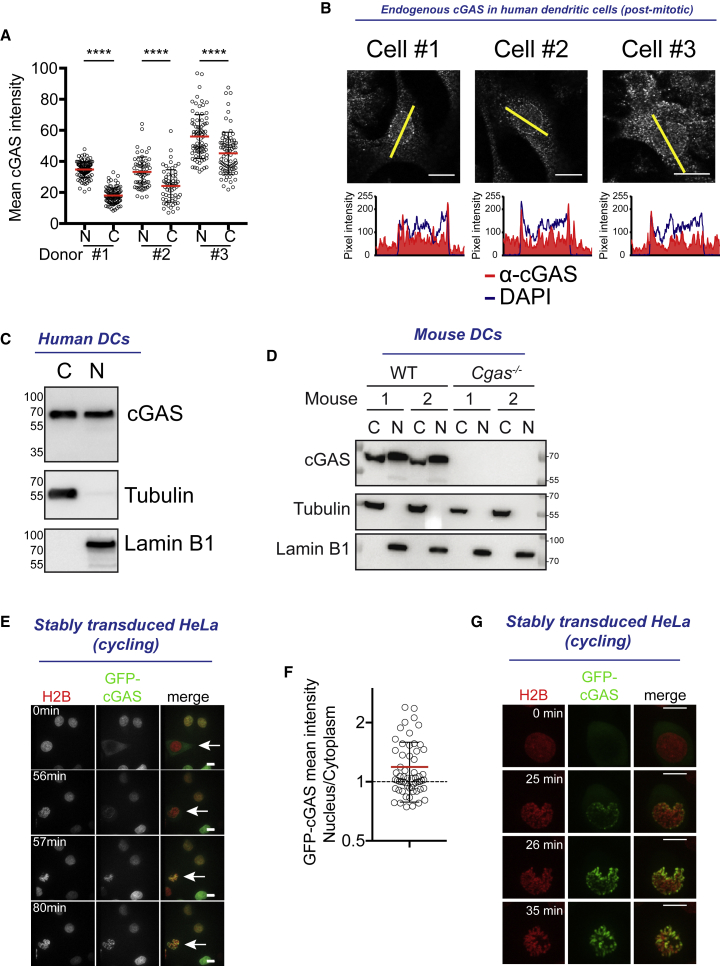
cGAS Is Present in the Nucleus as a Result of Nuclear Envelope Opening
(A) Quantification of mean endogenous cGAS intensity in the nucleus (N) or in the cytoplasm (C) of post-mitotic human monocyte-derived dendritic cells (DCs) (n > 60 cells for each donor, 3 independent donors combined from 2 independent experiments; red lines represent average and black lines represent SD, 1-way ANOVA with post hoc Tukey test; ∗∗∗∗p < 0.0001).
(B) Top: immunofluorescence staining of endogenous cGAS (red) and DAPI (blue), cGAS staining and (bottom) overlay plots of pixel intensity measured along the yellow line of cGAS (red) and DAPI (blue). For DAPI, refer to Figure S1B. Scale bars, 10 μm.
(C) Nuclear-cytoplasmic fractionation of post-mitotic human DCs and immunoblots for endogenous cGAS (top), tubulin (center), and lamin B1 (bottom). C, cytosolic fraction; N, nuclear fraction. One donor representative of n = 4 donors. See Figure S1C for the other donors.
(D) Nuclear-cytoplasmic fractionation of mouse bone marrow-derived DCs from two wild-type (WT) or two cGAS knockout (Cgas−/−) mice and immunoblot for endogenous cGAS (top), tubulin (center), and lamin B1 (bottom). C, cytosolic fraction; N, nuclear fraction (representative of n = 3 independent mice).
(E) Sequential images of cycling HeLa cell stably expressing histone 2B (H2B)-mCherry (red) and GFP-cGAS (green) before (0 min), at (56–57 min), and after (80 min) nuclear envelope breakdown. Scale bars, 10 μm.
(F) Nuclear-cytoplasmic ratio of mean GFP-cGAS intensities in cells as in (E) (n = 59 cells combined from 2 independent experiments; red line represents mean, error bars represent SDs).
(G) Sequential images of one representative HeLa cell as in (E) with GFP-cGAS in the cytosol before mitosis. Scale bars, 10 μm.
See also Figure S1.