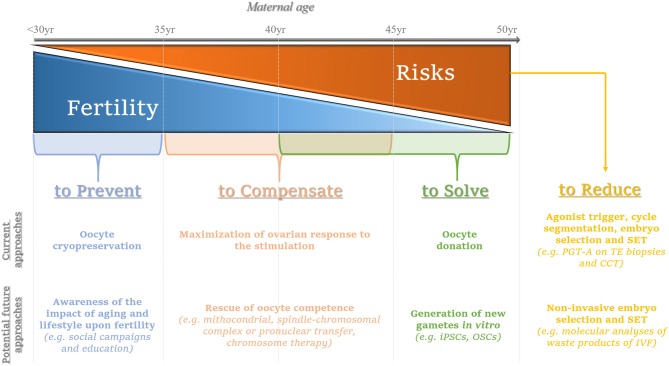
Figure 1.
Summary of the current and potential future approaches to prevent, compensate or solve the issues related with advanced maternal age on infertility, while limiting the putative concurrent risks. The consequences deriving from the onset of aging-related female infertility might be prevented by performing oocyte cryopreservation ideally when women are younger than 35 year; in the future, social campaigns and education are advisable to promote the awareness of the age-related female fertility decay. The main current strategy to compensate for the consequences of aging on oocyte competence (woman age range: 35–45 year) entails the maximization of the ovarian response by tailoring patients-specific protocols; in the future, oocyte competence might be rescued via promising approaches such as mitochondrial, spindle-chromosomal complex, pronuclear transfer or chromosome therapy, even though all these perspectives still need thorough and careful investigation. Oocyte donation represents the main current option to solve irrecoverable aging-related infertility conditions (woman age range: 40–50 year); the future avant-gardes that are motivating the academic research instead entail the generation of new gametes in vitro [e.g., from induced pluripotent stem cells (iPSCs) or oogonial stem cells (OSCs)]: an intriguing challenge with yet unpredictable outcomes. At last, the increasing maternal age brings about greater reproductive risks for the woman, the pregnancy she will eventually achieve and the new-born: to date, embryo selection via preimplantation genetic testing of aneuploidies (PGT-A) performed by molecular platforms for comprehensive chromosome testing (CCT) on trophectoderm (TE) biopsies represents the most efficient workflow to counteract the hazards derived from embryo chromosomal aneuploidies. Agonist trigger and cycle segmentation are important to minimize the risk of ovarian hyperstimulation syndrome (OHSS) after IVF. Moreover, to avoid the onset of multiple gestations, single embryo transfer (SET) is strongly recommended, especially for euploid blastocysts. In the future, molecular analyses (DNA, mRNA, miRNA, and/or proteins) on leftover products of IVF retrieved via non-invasive procedures (e.g. cumulus cells, spent culture media after IVF) might be implemented clinically to complement (or even replace) invasive approaches of embryo selection thereby aiming at a successful SET. From left to right, the gray arrow represents the increasing maternal age from <30 to 50 year The blue triangle depicts the decreasing maternal fertility, while the orange triangle depicts the increasing risks that could derive from the treatment of infertility and/or the establishment of a pregnancy as the maternal age increases. In light blue, the current and emerging approaches to prevent the fertility decay. In light orange, the current and emerging approaches to compensate it. In green, egg donation is proposed as a current strategy to circumvent the aging issue of female gametes, while emerging still experimental approaches aim at solving this issue. In yellow, we summarized the current strategies recommended to limit the putative risks of an IVF treatment; in the future, non-invasive approaches for embryo selection might be implemented in this workflow to identify the embryo for SET.