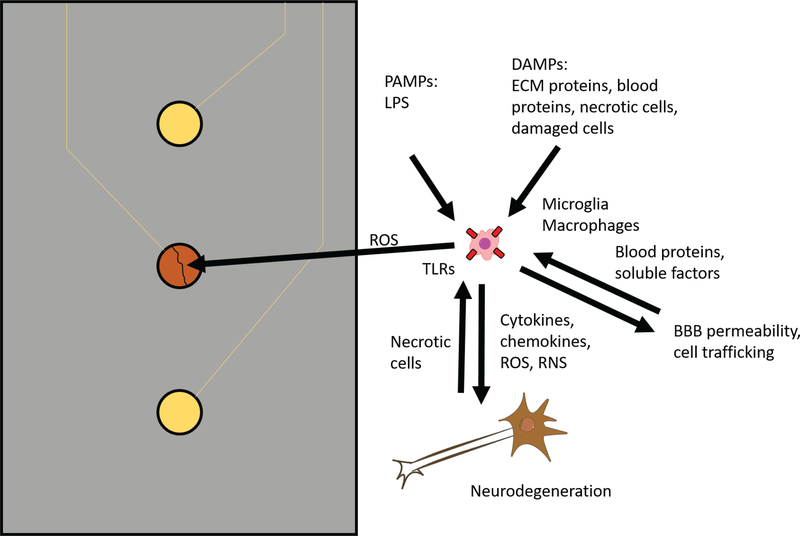
FIG. 2:
Potential role of TLR2, TLR4, and CD14 in the foreign body response to intracortical microelectrodes. Microglia and macrophages at the electrode tissue interface may recognize PAMPs and DAMPs at the electrode tissue interface via TLR2, TLR4, and CD14, resulting in the release of soluble factors such as cytokines, chemokines, reactive oxygen species, and reactive nitrogen species. The released soluble factors may damage neurons, blood vessels, and electrode materials, leading to poor intracortical microelectrode performance. Cells damaged and killed by the soluble inflammatory factors may release DAMPs that activate microglia and macrophages. Blood proteins released following damage to the blood brain barrier may also act as DAMPs. Inflammatory cells infiltrating across the blood-brain barrier permeability may become activated by DAMPs and contribute to the foreign body response. Thus, TLR2, TLR4, and CD14 signaling may contribute to the activation and perpetuation of chronic inflammatory mechanisms in the foreign body response to intracortical microelectrodes.