Figure 2.
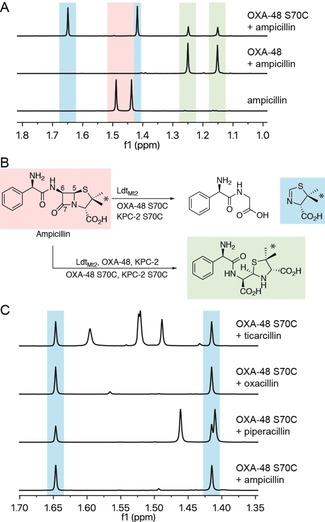
Reaction of penicillins with LdtMt2 and SBL thiol variants. A) 1H NMR spectra (700 MHz) of methyl group resonances for products formed upon addition of ampicillin to OXA‐48 S70C, compared to wild‐type OXA‐48. These signals correspond to methyl groups indicated by the asterisks in panel B. B) Scheme showing ampicillin‐derived fragmentation products formed by LdtMt2 and SBL thiol variants, and hydrolysis products formed by wild‐type SBLs, SBL thiol variants, and LdtMt2. C) 1H NMR spectra (600 MHz) showing the formation of the same dihydrothiazole fragmentation product upon incubation of OXA‐48 S70C with ampicillin, piperacillin, oxacillin, and ticarcillin (see also Figure S8 in the Supporting Information). Note the other signals present arise from intact penicillins and hydrolyzed products.
