Figure 4.
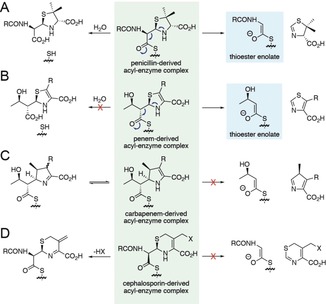
Comparison of the reactivities of the acyl‐enzyme complexes formed from LdtMt2 with different β‐lactam antibiotic sub‐classes. For the A) penicillins and B) penems, C5–C6 fragmentation gives rise to a thioester‐enolate. Note that penicillin hydrolysis occurs preferentially to fragmentation for LdtMt2 (Figure 2 and Supporting Information, Figure S8), while only fragmentation of faropenem was detected (Figure 3 and Supporting Information, Figure S11). C) C5–C6 fragmentation was not observed for the carbapenems (Supporting Information, Figure S1), potentially in part due to delocalization of the pyrroline nitrogen lone pair or due to tautomerization of the pyrroline ring (as well as the lack of a heteroatom at position 1 of the ring). Note the hypothetical product would be expected to tautomerize to a pyrrole. D) C6–C7 fragmentation was not observed for cephalosporins; however, elimination of the C3′ leaving group occurs (Supporting Information, Figure S1). Note that carbapenem and cephalosporin hydrolysis by LdtMt2 was not observed under our NMR conditions.
