Figure 13.
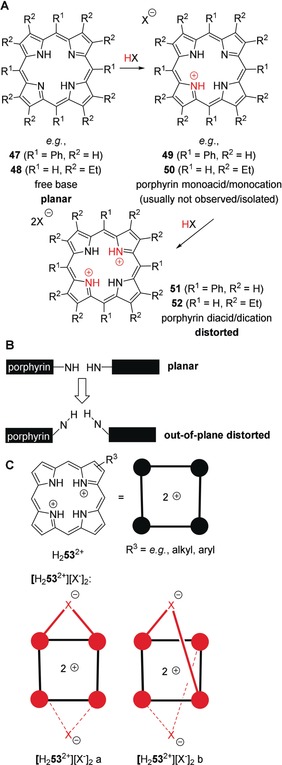
A: Protonation of free base‐porphyrins, for example, 5,10,15,20‐tetraphenylporphyrin (H2TPP, 47) or 2,3,7,8,12,13,17,18‐octaethylporphyrin (H2OEP, 48) by acids HX results in the formation of porphyrin dications and their salts, for example, [H4TPP2+][X−]2 (51) or [H4OEP2+][X−]2 (52). This proceeds via a monocation, for example, 49 or 50, which cannot usually be isolated. X−=acid anion. B: Illustration of pyrrole out‐of‐plane distortion in porphyrins/porphyrin core acids. C: Porphyrin acid salts, for example, [H2 53 2+][X−]2 are H‐bonding complexes where various conformations, such as [H2 53 2+][X−]2 a and b are conceivable.
