Abstract
Older adults typically show decline in a variety of cognitive functions including inhibitory control and language production, with corresponding age-related increases in fMRI activation. However, it remains unclear whether such increases are compensatory or whether they reflect neural decline. One factor that may influence these brain-behavior relationships is difficulty. The current study investigated the effect of difficulty on age-related differences in the behavioral and neural bases of language production and inhibitory control using a phonological Go/No-Go picture naming task. Task demands were manipulated by varying the proportion of naming trials (Go trials) and inhibition trials (No-Go trials) across runs. All participants showed task-difficulty related declines in behavioral performance and increases in fMRI activation. Behaviorally, older adults were more sensitive to task difficulty, and elicited more fMRI activation than younger adults. Older adults were less neurally responsive to additional task demands (i.e., picture naming alone vs. Go/No-Go picture naming), but interestingly showed similar within-task increases as younger adults (e.g., Go Bias vs. No-Go Bias). Moreover, the relationships between fMRI activation and behavioral performance in older adults were multifaceted and the strength of these relations changed as a function of task difficulty. Specifically, activation in pre- and post- central gyri, right supramarginal and angular gyri was negatively correlated with naming reaction times, suggesting that activation in these regions may help mitigate age-related declines in language production. These findings are partially consistent with the CRUNCH model, highlighting the important influence of task difficulty on older adults’ behavioral performance and their patterns of fMRI activation during language production.
Keywords: Language production, Inhibitory control, Aging, Task difficulty, fMRI
1. Introduction
Older adults typically exhibit age-related decline in language production when compared to younger adults (Burke & Shafto, 2008; Diaz, Rizio, & Zhuang, 2016). For example, older adults experience more tip-of-the-tongue (TOT) phenomena, in which they are temporarily unable to produce a target word (e.g., Burke, MacKay, Worthley, & Wade, 1991; Shafto, Burke, Stamatakis, Tam, & Tyler, 2007); they are slower to name pictures (Mitchell, 1989; Morrison, Hirsh, & Duggan, 2003), have more slips of the tongue and misspellings (MacKay & James, 2004; Taylor & Burke, 2000), and report fewer responses in verbal fluency tasks (Burke & Shafto, 2008; Troyer, Moscovitch, Winocur, Alexander, & Stuss, 1998). Moreover, older adults’ speech is sometimes syntactically simpler and less fluent than younger adults’ speech, which may be related to concomitant age-related declines in working memory (Kemper, Herman, & Lian, 2003; Kemper, Thompson, & Marquis, 2001; But see Nippold, Cramond, & Hayward-Mayhew, 2014).
Several theories have been proposed to account for age-related differences in cognition. The Inhibition Deficit Theory (IDT, Hasher, Stoltzfus, Zacks, & Rypma, 1991; Hasher & Zacks, 1988; Lustig, Hasher, & Zacks, 2007) proposes that as people age, it becomes harder to inhibit information, and this excess information can become distracting to older adults. Moreover, age-related differences in aspects of executive function, such as inhibition and selection, may be critical in understanding declining language production abilities. For example, if irrelevant lexical information is active during object naming, that information may compete for selection with the intended target. Consistent with this, older adults have been shown to produce more off topic speech in conversation (Arbuckle & Gold, 1993; Arbuckle, Nohara-LeClair, & Pushkar, 2000), although their conversation goals may also differ (Mortensen, Meyer, & Humphreys, 2008).
In contrast to the IDT, the Transmission Deficit Theory (TDT, Burke, MacKay, & James, 2000; Burke et al., 1991; MacKay & Burke, 1990) was specifically developed to account for age-related differences in language. Based on the Node Structure Theory (MacKay & Burke, 1990), the TDT proposes that connections among nodes are weakened by infrequent use and aging. Although all connections weaken with age, phonological processes are affected more extensively than semantic processes because there are fewer converging connections between phonological nodes compared to semantic nodes. These weaker, more divergent phonological connections lead to increased word retrieval failures for older compared to younger adults (Burke & Shafto, 2008). Consistent with this, older adults experience an increased number of Tip of the Tongue episodes (TOTs), where the speaker knows what they would like to say, but is temporarily unable to retrieve the item’s label (R. Brown & McNeill, 1966). Additional work by Burke and colleagues (1991) demonstrated that providing phonologically related words helped older adults resolve TOTs, further suggesting that the nature of the retrieval deficit is phonologically-based.
In addition to age-related declines in language production, age-related declines in brain structure are also commonly observed. Older adults typically show smaller grey matter volume and lower white matter integrity compared to younger adults, particularly in frontal and parietal regions (Good et al., 2002; Madden et al., 2012; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003). Despite these neural declines, older adults often exhibit increases in functional activation compared to younger adults, particularly in frontal cortex (e.g., Cabeza, 2002; Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Diaz, Johnson, Burke, & Madden, 2014; Diaz, Johnson, Burke, Truong, & Madden, In Press; Meinzer et al., 2009; Persson et al., 2004). Within the realm of language production, all adults engage a left-lateralized network including left inferior frontal gyrus; anterior, middle and superior temporal cortices; angular gyrus, and supramarginal gyrus (Binder, Desai, Graves, & Conant, 2009; S. Brown et al., 2009; Hickok & Poeppel, 2007; Hirshorn & Thompson-Schill, 2006; Indefrey & Levelt, 2000, 2004; Mirman et al., 2014; Pobric, Jefferies, & Ralph, 2007; Poldrack et al., 2001; Price, 2010; Visser, Jefferies, & Ralph, 2010), however older adults often show less lateralized patterns of activation compared with younger adults (Destrieux et al., 2012; Diaz et al., 2014; Diaz et al., In Press; Diaz et al., 2016; Meinzer et al., 2009; Meinzer et al., 2012; Persson et al., 2004). For instance, during successful picture naming, older adults showed greater activation, not only within typical language networks, but also in regions related to executive function, such as the bilateral anterior cingulate, and bilateral inferior frontal and insular cortices (Wierenga et al., 2008).
Although age-related increases in fMRI activation have been consistently observed across multiple cognitive domains, it remains unclear whether such increases are compensatory (Cabeza, 2002; Davis et al., 2008; Reuter-Lorenz & Cappell, 2008) or whether they reflect neural decline, such as dedifferentiation or disinhibition (Ghisletta & Lindenberger, 2003; Li, Lindenberger, & Sikström, 2001). Compensatory accounts generally argue that age-related increases in activation via recruitment of additional regions serve to compensate for neural decline elsewhere. For example, some evidence suggests that increased activation in right inferior frontal gyrus, which has been associated with executive function, helps older adults maintain performance (e.g., Persson et al., 2004; Wierenga et al., 2008). Dedifferentiation accounts generally argue that age-related increases in activation reflect lower levels of inhibition and contribute to a noisier signal overall. Research in support of this latter interpretation typically either finds no relationship between increased right hemisphere activation and performance or that increased activation is associated with declines in behavioral performance, suggesting that the increases in activation reflect less efficient processing (Diaz et al., 2014; Diaz et al., In Press; Meinzer et al., 2009; Meinzer et al., 2012). One factor that may influence age-related differences in brain activation and brain-behavior relationships is task difficulty. For instance, studies have shown that older adults use different strategies to accommodate task demands, suggesting that younger and older adults experience and cope with changes in task difficulty differently (Kemper, Schmalzried, Herman, Leedahl, & Mohankumar, 2009; Kemper, Schmalzried, Herman, & Mohankumar, 2011). The CRUNCH model (Compensation-Related Utilization of Neural Circuits Hypothesis, Reuter-Lorenz & Cappell, 2008) proposes that as task demands initially increase, older adults will show larger increases in brain activation and larger impairments in behavioral performance compared to younger adults. The CRUNCH model hypothesizes that at these lower levels of task difficulty, the recruitment of additional brain regions in older adults may initially help individuals maintain behavioral performance, supporting compensation accounts. However, as task demands increase and begin to exceed cognitive resources, brain activation may start to decline and the correlations between cortical fMRI activation and behavior may be reduced or become maladaptive, in line with dedifferentiation accounts. Thus, this hypothesis proposes an inverted U-shaped function for brain activation as task difficulty increases and exceeds their available resources. Although this general neural and behavioral response to task difficulty may be found across the lifespan, dissociations between activation and performance may occur at lower levels of task difficulty for older adults due to age-related neural decline. This model provides a promising explanation of age-related fMRI over-recruitment. Yet, to our knowledge, few studies have applied the CRUNCH model to language production. Therefore, the goal of the present study was to explore the behavioral and neural bases of language production and task demands in older and younger adults during a phonological Go/No-Go picture naming paradigm.
The Go/No-Go paradigm is widely used as a way to measure response inhibition, which involves a set of cognitive functions including response control, attentional monitoring, working memory, and global proactive control (Aron, 2011; Simmonds, Pekar, & Mostofsky, 2008; Wijeakumar et al., 2015). Typically, in a Go/No-Go paradigm, participants respond to a particular type of stimuli (Go trials) and withhold their responses to other types of stimuli (No-Go trials). Research has shown that a right lateralized fronto-striatal network (including dorsal lateral prefrontal cortex, inferior frontal gyrus/insula, precentral gyrus and pre-supplementary motor area extending to anterior cingulate cortex, basal ganglia, and inferior parietal regions) is sensitive to these response inhibition processes (Aron, 2011; Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004; Buchsbaum, Greer, Chang, & Berman, 2005; Criaud & Boulinguez, 2013; Garavan, Ross, & Stein, 1999; MacDonald, Cohen, Stenger, & Carter, 2000; Nieuwenhuis, Yeung, Van Den Wildenberg, & Ridderinkhof, 2003; Ridderinkhof, Van Den Wildenberg, Segalowitz, & Carter, 2004; Simmonds et al., 2008). Response inhibition demands in the Go/No-Go paradigm can be manipulated by altering the proportion of Go and No-Go trials to create a response bias. For example, when there are few No-Go trials (e.g., 20%), response inhibition demands are higher compared to conditions where No-Go trials are equiprobable. Similarly, for Go trials, execution of the behavior is harder when there are fewer Go trials compared to runs when there are more Go trials and rare trials entail higher attentional demands, irrespective of inhibitory requirements (Wijeakumar et al., 2015). Moreover, the proportion manipulation of Go/No-Go trials always affects both response execution and inhibition because reduction in one condition is directly tied to an increase in the other.
In the present study, we examined how task demands affect language production in both younger and older adults by incorporating the Go/No-Go paradigm with a commonly used language production task: Picture Naming. One advantage of using the Go/No-Go paradigm is that language production and response inhibition can be investigated interactively. Some studies have incorporated the Go/No-Go paradigm with language production using electrophysiology (Rodriguez-Fornells, Schmitt, Kutas, & Münte, 2002; Schmitt, Rodriguez-Fornells, Kutas, & Münte, 2001; Van Turennout, Hagoort, & Brown, 1997) or fMRI (Rodriguez-Fornells et al., 2005; Zhang, Eppes, Beatty-Martinez, Navarro-Torres, & Diaz, 2018). However, no studies to date have examined these issues with older adults. In the current study, we used phonological aspects of photographs as the cues to make Go/No-Go decisions (e.g., name if the photograph’s name started with a consonant, and withhold a response if the photograph’s name started with a vowel). Three levels of naming demands were included. First, to be comparable with previous studies using the picture naming paradigm, we included runs in which participants named all the photographs (i.e., lowest naming difficulty). Second, we included runs that contained a majority of Go trials (naming trials, e.g., 75%) and infrequent No-Go trials (withhold naming trials, e.g., 25%). These runs had low picture naming demands, but high response inhibition demands for the No-Go trials. Third, we incorporated runs that involved a majority of No-Go trials (withhold naming trials, e.g., 75%) and infrequent Go trials (naming trials, e.g., 25%). Correspondingly, these runs had lower response inhibition demands during the No-Go trials, but higher picture naming demands during the naming trials.
We predicted that older and younger adults’ behavioral and neural responses would differ as a function of task demands in the phonological Go/No-Go picture naming paradigm. Specifically, older adults would show declines in behavioral performance (i.e., longer reaction times, more errors) and elicit less lateralized patterns of activation, particularly in frontal regions, compared to younger adults. Moreover, we predicted that increases in naming difficulty would elicit more activation in language regions that are sensitive to task demands, such as left inferior frontal gyrus, as well as those sensitive to phonological processing such as posterior superior temporal sulcus, supramarginal gyrus, and pre- and post-central gyri (Novick, Trueswell, & Thompson‐Schill, 2010; Peramunage, Blumstein, Myers, Goldrick, & Baese-Berk, 2011; Roskies, Fiez, Balota, Raichle, & Petersen, 2001; Schnur et al., 2009). The increases in naming difficulty (as the number of Go trials decrease) could also elicit greater activation in regions sensitive to attentional demands such as bilateral insula/inferior frontal gyrus, right putamen and thalamus (Wijeakumar et al., 2015). Similarly, response inhibition difficulty during No-Go trials should also change with the proportions of No-Go trials in a run. For the No-Go trials, we hypothesized that inhibition failures and activation in supplementary motor cortex extending to anterior cingulate and right prefrontal cortex would increase as a function of inhibition difficulty (Botvinick et al., 2001; Botvinick et al., 2004; Criaud & Boulinguez, 2013). Furthermore, based on the CRUNCH model, we predicted that older adults would show greater changes in behavioral performance as a function of task difficulty, compared to younger adults. In terms of brain activation, we expected older adults to be less neurally responsive to task-difficulty manipulations. This could manifest as either a flat response (vs. linear increases) or an inverted U response with initial increases in BOLD activation followed by decreases due to reaching their resource ceiling. Additionally, we predicted that older adults’ brain activation would help their behavioral performance when task demands were low, and that this compensatory pattern may not hold as task demands increase.
2. Method
2.1. Participants
Twenty younger adults (ages: 18–34, mean age = 22.65 years, 10 female) and 20 older adults (ages: 61–79, mean age = 67.45 years, 14 female) participated in the experiment. Results for the younger adults were previously published (Zhang et al., 2018), so here we focus on age-related differences. All participants were community-dwelling, right-handed, native English speakers who were not fluent in a second language. All participants had normal or corrected-to-normal vision, and reported no history of neurological, psychological, or major medical conditions (Christensen, Moye, Armson, & Kern, 1992). Prior to the MRI session, each participant completed a battery of psychometric and neuropsychological tests to assess basic cognitive functions such as speed, working memory, executive function, and language. These tasks included the Mini-Mental Status Exam to screen for mild cognitive impairment or dementia (MMSE, Folstein, Folstein, & McHugh, 1975); WAIS-III vocabulary and digit-symbol subtests (Wechsler, Coalson, & Raiford, 1997); phonemic (F, A, S) and categorical (animals) verbal fluency; the author recognition and magazine recognition tests to assess reading habits (Acheson, Wells, & MacDonald, 2008); the California Verbal Learning Test to assess immediate and delayed memory (Woods, Delis, Scott, Kramer, & Holdnack, 2006); simple and choice reaction time tests to assess speed; forward and backward digit span to assess working memory; the AX version of the continuous performance task (AX-CPT, Braver et al., 2001; Braver, Gray, & Burgess, 2007), the Stroop task (MacLeod, 1991; Stroop, 1935), and a task-switching task to measure executive function (Monsell, 2003). Across groups, participants did not differ in years of education, vocabulary, verbal fluency, recall, digit span, or task switching. Demographic characteristics and assessment scores are reported in Table 1. All participants gave written, informed consent, and all procedures were approved by the Institutional Review Board at The Pennsylvania State University.
Table 1.
Participant demographic and neuropsychological testing scores
| Younger Adults | Older Adults | |
|---|---|---|
| Demographic information | ||
| N | 20 | 20 |
| Age* | 22.65 (4.3) | 67.45 (5.75) |
| Gender (M/F) | 10/10 | 6/14 |
| Education (Years) | 16.1 (2.36) | 16.41 (2.74) |
| Cognitive Assessment | ||
| Mini-Mental State Examination (Score out of 30)* | 29.05 (1) | 28.35 (1.09) |
| WAIS Vocabulary (Score out of 66) | 49.8 (9.6) | 53.11 (6.25) |
| Verbal Fluency (Total Score) | 56.95 (18.67) | 55.2 (19.57) |
| Author Recognition (Total Score)1* | 13.1 (8.64) | 30.63 (13.81) |
| Magazine Recognition (Total Score)1* | 12.7 (7.76) | 27.11 (3.77) |
| Immediate Recall (Score out of 16) | 11.25 (2.15) | 10.6 (2.48) |
| Delayed Recall (Score out of 16) | 10 (2.29) | 8.55 (2.74) |
| Simple Speed (ms)* | 253.44 (27.25) | 281.56 (50.77) |
| Complex Speed (ms)* | 269.44 (20.16) | 317.29 (30.46) |
| Digit Span Forward (Score out of 30) | 10.65 (1.39) | 11.05 (1.85) |
| Digit Span Backward (Score out of 30) | 7 (1.69) | 6.85 (1.98) |
| Digit Symbol (ms)* | 1298.83 (156.4) | 1823.08 (284.78) |
| AX-CPT: AY RT (ms)* | 386.03 (47.71) | 483.92 (82.29) |
| AX-CPT: AY ER | 0.27 (0.20) | 0.23 (0.15) |
| Stroop Effect (ms) (Incongruent – Congruent)* | 40.24 (75.55) | 113.21 (132.48) |
| Task Switching Effect (Switch - Nonswitch) | 56.33 (73.03) | 92.79 (89.89) |
Values provided are means, with standard deviation in parentheses.
Denotes a statistically significant difference, p < .05.
ART and MRT scores are calculated as the number of correct identifications – the number of incorrect responses.
2.2. Stimuli and procedure
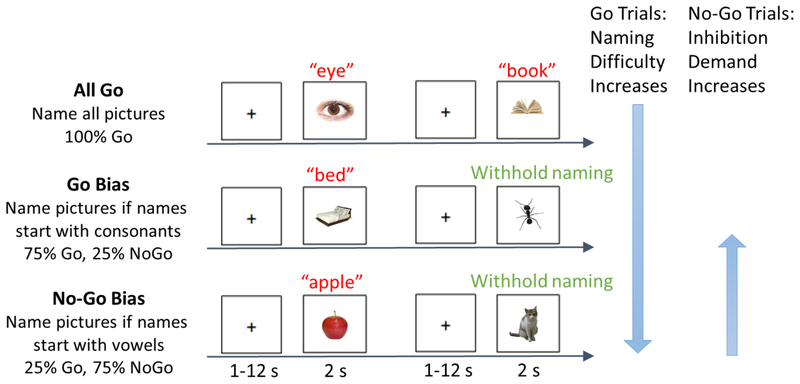
Participants performed a phonological Go/No-Go picture naming task in the scanner. Photographs were presented one at a time and participants were instructed to overtly name the photograph as quickly as possible while still responding accurately. Task demands were manipulated via the proportion of trials that needed to be named or inhibited, constituting three conditions: All Go, Go Bias, No-Go Bias (Figure 1, reproduced with permission from Zhang et al., 2018). In the All Go condition, participants were instructed to name all of the photographs. In the Go Bias condition (75% Go trials, 25% No-Go trials), participants were required to name the photograph if the name of the photograph started with a consonant (i.e., Go trials, e.g., nose) and to withhold their response if the name started with a vowel (i.e., No-Go trials, e.g., apple). In contrast, in the No-Go Bias condition (25% Go trials, 75% No-Go trials), participants were instructed to name the photograph if the name started with a vowel (i.e., Go trials, e.g., orange) and to withhold their response if the name started with a consonant (i.e., No-Go trials, e.g., chair).1
Figure 1.
Task design (reprinted by permission from Springer Nature: Cognitive, Affective, & Behavioral Neuroscience, Zhang et al., 2018). An overview of the phonological Go/No-Go picture naming task is provided. Examples of Go trials and No-Go trials for each of the three conditions: All Go, Go Bias, and No-Go Bias. Correct names to the two No-Go trials (noted in green) are “ant”, and “cat”, respectively. Naming (Go trials, in red) difficulty increased from the All Go condition to the Go Bias condition to the No-Go Bias condition. Inhibition (No-Go trials) demand increased from the No-Go Bias condition to the Go Bias condition.
Prior to scanning, participants practiced overt picture naming while minimizing head movement in a mock scanner. In the scanner, participants always performed the All Go condition first, prior to being informed about the Go/No-Go manipulation to avoid naming biases in this first run. After the All Go condition, participants underwent a practice run and then completed the Go Bias and the No-Go Bias conditions, whose order was counterbalanced across participants2. Photographs were not repeated across practice runs or conditions.
Photographs were taken from two normed databases (Brodeur, Guérard, & Bouras, 2014; Moreno-Martínez & Montoro, 2012). These images depicted common concrete objects from a variety of categories such as animals, clothing, food, and household items. In developing the final experimental stimuli, a norming study was conducted to confirm the naming consistency of these photographs. An independent group of 21 healthy, native English-speaking younger adults named 592 color photographs. Items were included in the final stimulus set only if naming accuracy was 67% or higher (mean accuracy = 87.74%, SD = 10.42%). The final set of stimuli for the MRI experiment included 330 colored photographs, 110 unique items per condition. For the Go Bias and No-Go Bias conditions, these trials were further divided into 82 trials (75%) of the biased trial type (e.g., Go trials in the Go Bias runs) and 28 trials (25%) of the non-biased trial type (e.g., No-Go trials in the Go Bias runs). Linguistic characteristics for all of the final stimuli were obtained from the English Lexicon Project (ELP, Balota et al., 2007, see Supplemental Table 1 for all word characteristics). The names of photographs across the three conditions were matched according to word length (F (2, 327) = .75, p = .47), word frequency (F (2, 327) = .17, p = .84), number of phonemes (F (2, 327) = .30, p = .74), number of syllables (F (2, 327) = .33, p = .72), and reaction time (RT) and accuracy based on the ELP data (Lexical Decision Task (F1 (2, 327) = .72, p1 = .49; F2 (2, 327) = 1.43, p2 = .24); Word Naming Task (F1 (2, 327) = .44, p1 = .65; F2 (2, 327) = .69, p2 = .50))3.
In each trial, one color photograph (396 pixels × 396 pixels) was presented on a white background and participants were instructed to respond with the target name or withhold their response based on the condition requirements. Participants were also asked to limit their answer to only one word. Photographs (duration = 2 s) were presented with a variable inter-stimulus interval (range = 1–12 s, mean = 3.40 s) that was determined using the optseq2 program, as jittered presentations have been shown to optimize the hemodynamic response (Dale, 1999) and prevent participants from anticipating the onset of events. Participants completed 6 runs (2 runs per condition) in the scanner. During the task, overt verbal responses were recorded and filtered using an MR-compatible fiber optic microphone system (Optoacoustics Ltd., Or-Yehuda, Israel). To verify participants’ identification and naming of the photographs, after the scan they were asked to name all of the photographs from the Go Bias and the No-Go Bias conditions.
2.3. Acquisition of MRI data
MRI scanning was completed on a 3T Siemens Prisma Fit MRI scanner with a 20-channel head coil. Sagittal T1 weighted localizer images were collected and used to define a volume for data collection, higher-order shimming, and alignment to the anterior commissure and posterior commissure (AC-PC). T1 weighted anatomical images were collected using a magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence (repetition time [TR] = 2300 ms; echo time [TE] = 2.28 ms; Inversion Time [TI] = 900 ms; flip angle = 8°; echo spacing = 7 ms; acceleration factor = 2; field of view [FOV] = 256 mm2; voxel size = 1 × 1 × 1 mm; 160 contiguous slices).
Functional images were collected using an echo-planar imaging (EPI) sequence (TR = 2500 ms; TE = 25 ms; flip angle = 90°; echo spacing = 0.49 ms; FOV = 240 mm2; voxel size = 3 × 3 × 3 mm; 41 contiguous axial slices, parallel to the AC–PC, interleaved acquisition, 122 volumes (305 s) per run). Two additional volumes were acquired and deleted at the beginning of each functional run to reach steady state equilibrium.
2.4. Behavioral Data Analyses
Responses were coded based on both the recordings from the scanner session and the post-scan naming task. In the All Go condition, responses were marked as correct if the participant provided the exact target name (e.g., chicken for chicken), plural form of the photograph name (e.g., chickens for chicken), or an acceptable alternative word that corresponded to the photograph (e.g., hen for chicken). Responses were marked as incorrect if the response did not match the photograph (e.g., ice for tea), or if no response was provided (i.e., omission errors).
For Go trials in the Go Bias and the No-Go Bias conditions, responses were marked as correct as indicated above, with the caveat that an acceptable alternative that matched the photograph also had to have the same onset category (vowel/consonant) as the target word (e.g., raven for crow would be fine but not spaceman for astronaut). For No-Go trials in the Go Bias and the No-Go Bias condition, they were marked as correct if no response was provided in the scanner and the post-scan naming task indicated that the participant knew the name of the photograph.
Errors were coded as three types for the Go Bias and the No-Go Bias conditions: 1) incorrect responses (e.g., Go trials: a response that did not match the picture, or had an incorrect onset category; No-Go trials: no response combined with not knowing the picture in the post-scan naming task or a response that had an incorrect onset category); 2) commission errors (failures to inhibit a response during a No-Go trial. Note that responses with incorrect onsets such as spaceman for astronaut were coded as ‘incorrect responses’ and not included in the commission error rates); and 3) omission errors (no response to a Go trial). Error rates in each condition were calculated by dividing the number of errors by the number of possible trials in that condition (e.g., total errors/total number of trials; commission errors/number of No-Go trials). The three types of errors were analyzed by treating the number of errors as a categorical variable using generalized logistic mixed-effect modeling, employing the glmer function in the lme4 package (Bates, Mächler, Bolker, & Walker, 2014) in the R environment (Venables & Smith, 2006). For each trial type, errors of that type were coded as 1s and other trials were coded as 0s. We obtained p values for regression coefficients using the lmerTest package (Kuznetsova, Brockhoff, & Christensen, 2017). This approach has the advantage of taking into account individual data points, allowing intercepts and slopes to be random across participants and allowing different items to have random intercepts. As recommended by Barr, Levy, Scheepers, and Tily (2013), linear mixed-effect models generalize best when they include the maximal random effects structure justified by the design. Therefore, for each type of error, a full model included task condition, age group, and their interaction as the predictors and the error (1 vs. 0) as the dependent variable, while allowing different participants to have random intercepts and slopes and different items to have random intercepts. To make the results more interpretable, age group was recoded using contrast coding (Younger is −0.5, and Older is 0.5). Additionally, for commission errors and omission errors, the task condition was also contrast coded (Go Bias is −0.5, No-Go Bias is 0.5). For commission errors, another model adding the error rates in the AY condition in the AX-CPT (an index of inhibition ability) as the participant level variable was also conducted to further validate our manipulation.
Reaction times (RTs) to Go trials were calculated using customized PRAAT scripts. The PRAAT scripts identified response onsets by searching the recordings for pitch deviations within the filtered auditory signal. These onsets were then manually verified by using both the audio and visual speech stream. The reaction times were calculated as the difference between the photograph onsets (from E-Prime output) and the response onsets. Only trials with correct responses and reaction times within 2.5 SDs were included in further analyses. Reaction times were analyzed using mixed-effect regression modelling, employing the lmer function in the lme4 package (Bates et al., 2014) in the R environment (Venables & Smith, 2006). To make results more interpretable, age group was recoded using contrast coding (Younger is −0.5, and Older is 0.5) and included as one numeric variable. Because task condition has three levels (i.e., the All Go condition and the two Bias conditions measuring naming difficulty), there was no straightforward way to contrast code all levels equally, in the same model. Therefore, pair-wise comparisons were contrast coded separately (All Go vs. Go Bias, Go Bias vs. No-Go Bias, All Go vs. No-Go Bias). Task condition and its interaction with age group were also included as other independent variables and reaction time was included as the continuous dependent variable, while allowing different participants to have random intercepts and slopes, and different items to have random intercepts.
2.5. fMRI data analyses
The fBIRN QA tool was used to assess data quality (Glover et al., 2012, https://www.nitrc.org/projects/bxh_xcede_tools/), measuring the number of potentially clipped voxels, mean signal fluctuation to noise ratio (SFNR), and per-slice variation. Additionally, the anatomical and functional images were visually inspected for artifacts and signal drop-out. Non-brain tissue of the anatomical images was removed using Optimized Brain Extraction for Pathological Brains (optiBET: Lutkenhoff et al., 2014). We used FSL (version 5.0.9), with FEAT (fMRI expert analysis tool) version 6.0 (Smith et al., 2004; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004), to carry out preprocessing and statistical analyses. Preprocessing steps included motion correction (FSL MCFLIRT), B0 unwarping, slice timing correction, spatial smoothing (FWHM = 5 mm), high-pass filtering, coregistration, and normalization. We used a double-gamma hemodynamic response function to model the BOLD signal for each event and only correct trials were included in the analyses. We conducted first level analyses on each participant’s individual runs, including the standard motion parameters as nuisance covariates. Analyses from previous steps were combined across participants in group-level analyses using FMRIB’s local analysis of mixed effects (FLAME 1+2, Beckmann, Jenkinson, & Smith, 2003; Woolrich et al., 2004). Because the All Go condition and Bias conditions differ in their task demands, our primary analytic approach was to analyze these runs separately. Therefore, for each age group, we first identified regions that were significantly activated during the All Go Condition and across the Go and No-Go trials in the Bias conditions compared with the implicit baseline, then looked for differences between age groups. We also examined the data for changes in functional activation as a function of task difficulty for Go trials (Go Bias < No-Go Bias) and No-Go trials (No-Go Bias < Go Bias), then looked for differences between age groups in these comparisons. We were also interested in how the difference in task demands (e.g., additional phoneme monitoring demands of the Bias conditions) affected brain activation for older and younger adults. Therefore, secondary analyses were conducted on functional activation to Go trials, comparing the All Go and Bias conditions (All Go < Bias conditions), and whether these differences varied by age group. All significant activations were determined using a two-step process in which Z (Gaussianised T/F) statistical images were initially thresholded at the voxel level (p < .01). Clusters of identified voxels were then corrected for multiple comparisons (p < .05, corrected) based on Gaussian random field theory (Worsley, 2001) in which each cluster’s estimated significance level was compared with the cluster probability threshold, and then only clusters whose estimated significance exceeded the threshold were included in the results (Hayasaka & Nichols, 2003). Additionally, results from comparisons between conditions or groups were masked to ensure that only differences based on significant positive hemodynamic responses were included in the analyses (e.g., an analysis comparing Older > Younger for Go trials would be masked to include only regions that were significantly activated in the Older adults Go trials analysis).
One of our main questions of interest was whether age-related increases in fMRI activation reflect compensation or neural dedifferentiation. As with our primary analysis strategy, we analyzed brain-behavior relationships separately for the All Go condition and the bias conditions. We first correlated older adults’ reaction time with their brain activation in the All Go condition. To further assess brain-behavioral correlations with inhibitory control, additional analyses were conducted to correlate older adults’ behavioral performance (i.e., reaction time for Go trials, commission error rate for No-Go trials) with their brain activation collapsed across Bias conditions. This approach allowed us to examine the relationship between overall behavioral performance and functional activation across individuals. Furthermore, to investigate how task difficulty contributed to older adults’ brain-behavior relations, we compared the strength of these correlations for each trial type (i.e., Go trials: Go Bias > No-Go Bias; No-Go trials: No-Go Bias > Go Bias). As a secondary analysis, we also compared the strength of older adults’ brain-behavior correlations between the All Go condition and the Bias conditions for the Go trials (All Go > Bias conditions), to explore whether increasing task demands influenced brain-behavior correlations. All reported brain regions were identified using the Harvard-Oxford Structural Atlas (Desikan et al., 2006). Coordinates are reported in MNI space, and results are overlaid on a representative brain in MNI space.
A necessity of the Go/No-Go paradigm is that the number of critical trials differs across conditions which creates a potential power confound. To address this concern, we conducted a second analysis in which we randomly selected an equal number of critical trials across conditions and statistically compared the analysis including all trials to the analysis including equal numbers of trials for each condition4.
3. Results
3.1. Behavioral Results
3.1.1. Reaction Times (RTs)
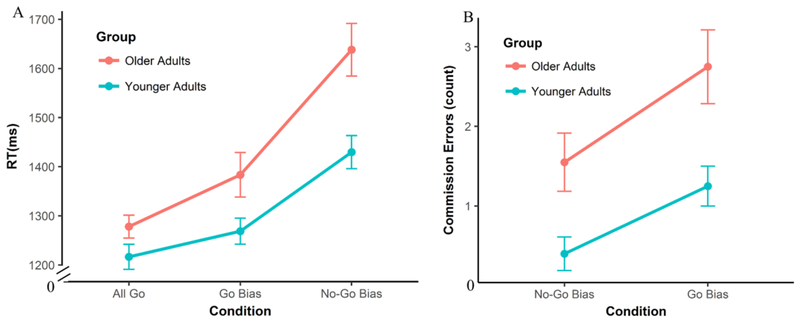
A generalized linear mixed-effect model was conducted on Go trial RTs to explore the differences across the three conditions (All Go, Go Bias, No-Go Bias) and the differences between the two age groups (Younger vs. Older). Results showed a main effect of condition — across both age groups, the RTs in the All Go condition were significantly shorter than the Go Bias condition (β = .07, SE = .03, p = .01) and the No-Go Bias condition (β = .30, SE = .04, p < .001), while the RTs in the Go Bias condition were also significantly shorter than the No-Go Bias condition (β = .22, SE = .04, p < .001). Furthermore, the effect of age group was significant on all three task conditions (All Go: β = .07, SE = .03, p = .05; Go Bias: β = .12, SE = .05, p = .02; No-Go Bias: β = .23, SE = .06, p = .002), as older adults consistently named pictures more slowly than younger adults. More importantly, the interaction between age group and condition was significant (β = .11, SE = .04, p = .008). To specify the interaction, a regression line was first fitted on reaction times as a function of task conditions for each participant (All Go < Go Bias < No-Go Bias), then an independent sample t-test was conducted on the subject-level regression coefficients between the two groups. Results indicated that the regression coefficients in older adults were significantly greater than younger adults (t (32.89) = 2.19, p = .04), indicating that older adults had larger increases in reaction times as naming demand increased (All Go < Go Bias < No-Go Bias)5. See Figure 2A and Supplemental Table 2 for more details.
Figure 2.
Behavioral results for the Go/No-Go picture naming task. A) Reaction Times (RTs) for Go trials. RTs significantly increased as naming difficulty increased. Older adults named pictures more slowly and had significantly greater increases in RT as difficulty increased compared to younger adults. B) Commission error counts for No-Go trials. The Go Bias condition elicited more commission errors than the No-Go Bias condition across groups, and older adults made more commission errors than younger adults. However, the interaction between Condition and Age Group was not significant.
3.1.2. Number of Errors
A mixed logistic regression was conducted on the number of overall errors to explore the general differences across the three conditions (All Go, Go Bias, No-Go Bias) and between age groups. Results showed that the overall error rates were not significantly different across conditions (All Go vs Go Bias: β = −.06, SE = .28, p = .83; All Go vs No-Go Bias: β = −.004, SE = .28, p = .99; Go Bias vs No-Go Bias: β = .05, SE = .27, p = .84). However, there was a main effect of age group where older adults made more errors than younger adults in all three task conditions (All Go: β = .45, SE = .21, p = .03; Go Bias: β = 1.14, SE = .21, p < .001; No-Go Bias: β = .99, SE = .20, p < .001) (See Supplemental Table 2 for details).
We also conducted a similar mixed-logistic regression on the number of commission errors. Results showed that there was a main effect of condition—the number of commission errors was significantly higher in the Go Bias condition than in the No-Go Bias condition (β = −2.22, SE = .38, p < .001). These results suggest that cognitive control demands in terms of response inhibition and attentional conflict monitoring were higher during the Go Bias condition, consistent with the intended effect of our response-bias manipulation. The error rates on the AY condition of the AX-CPT could be treated as a measure of inhibition (Braver et al., 2001; Braver et al., 2007). When adding it into the commission error regression model, it significantly predicted the number of commission errors across both conditions, suggesting that these two measures of response inhibition were consistent across individuals (β = 2.05, SE = .77, p = .008, positive correlation)6. Furthermore, older adults made significantly more commission errors than younger adults across both task conditions (β = 1.16, SE = .30, p < .001). However, the interaction between the age group and task condition was not significant (β = .56, SE = .59, p = .31, Figure 2B and Supplemental Table 2).
The mixed logistic regression on omission errors showed a main effect of condition – there were fewer omission errors during the Go Bias condition compared to the No-Go Bias condition (β = 1.97, SE = .39, p < .001), confirming that our manipulation biased participants toward not naming during the No-Go Bias condition. Furthermore, there was a main effect of age group – older adults made more omission errors than younger adults (β = 1.10, SE = .30, p < .001). However, the interaction between the age group and the task condition was not significant (β = .18, SE = .37, p = .63, See Supplemental Table 2 for details).
3.2. Neuroimaging Results
3.2.1. Go Trials: Effects of Age and Task Difficulty on Language Production
3.2.1.1. Basic Patterns of Activation and Main Effect of Age
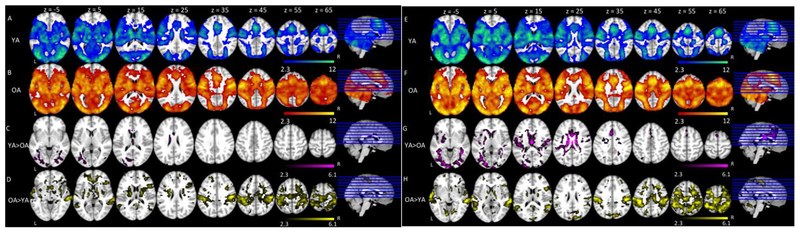
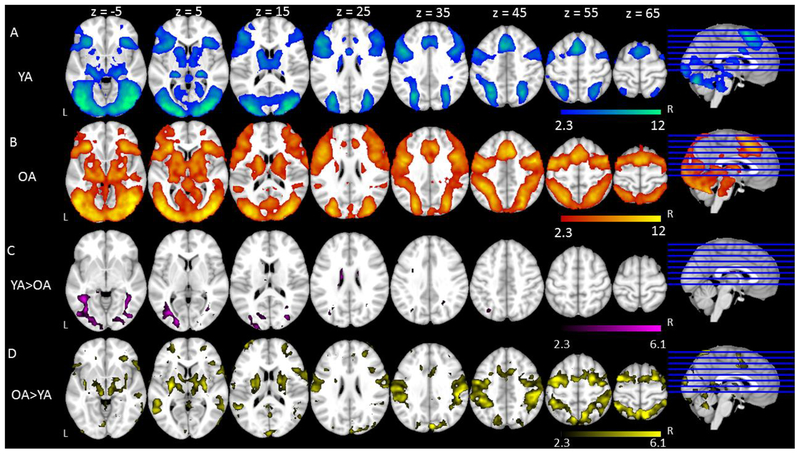
We first report the patterns of activation to Go trials in the All Go condition for both groups7. Across both age groups, Go trials elicited similar patterns of activation in established language-related regions that included bilateral middle and inferior frontal gyri, bilateral precentral and postcentral gyri, bilateral supramarginal gyri, and bilateral temporal cortex. Go trials also elicited activation in bilateral paracingulate gyri, bilateral anterior cingulate gyri, and bilateral occipital cortex (Figure 3A & 3B, Table 2). Comparing age groups, younger adults elicited greater activation than older adults in bilateral anterior cingulate gyri and occipital cortex (Figure 3C, Table 2). A comparison between age groups also showed that older adults elicited greater activation than younger adults in typical left hemisphere language regions and their right hemisphere homologues including bilateral superior and middle temporal gyri, bilateral supramarginal gyri extending to bilateral superior parietal lobe, bilateral pre- and post-central gyri, and bilateral lateral occipital cortex (Figure 3D, Table 2).
Figure 3.
Basic Patterns of Activation and Main Effect of Age on All Go condition and Bias conditions. Shown is an overview of the regions in which there was significant activation in (A) Younger Adults in All Go condition, (B) Older Adults in All Go condition, (C) Younger Adults > Older Adults in All Go condition, (D) Older Adults > Younger Adults in All Go condition, (E) Younger Adults in Bias conditions, (F) Older Adults in Bias conditions, (G) Younger Adults > Older Adults in Bias conditions, (H) Older Adults > Younger Adults in Bias conditions. Colored regions represent areas of statistically significant activation. Slices are depicted in increments of 10, starting at z = −5 and ending at z = 65.
Table 2.
Basic patterns of activation to Go trials in the All Go condition & Main effect of age group.
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Go Trials in the All Go Condition | ||||||
| Younger Adults | ||||||
| Frontal Pole | Left | 121888 | −36 | 40 | 18 | 3.32 |
| Frontal Pole | Right | 46 | 38 | 18 | 3.65 | |
| Inferior Frontal Gyrus | Right | 46 | 34 | 12 | 3.17 | |
| Inferior Frontal Gyrus | Left | −46 | 32 | 12 | 6.77 | |
| Anterior Cingulate Gyrus | Left | −10 | 28 | 20 | 3.68 | |
| Anterior Cingulate Gyrus | Right | 6 | 24 | 22 | 5.39 | |
| Middle Frontal Gyrus | Right | 44 | 22 | 24 | 2.3 | |
| Middle Frontal Gyrus | Left | −46 | 14 | 38 | 5.35 | |
| Precentral Gyrus | Left | −46 | −2 | 52 | 10.32 | |
| Precentral Gyrus | Right | 46 | −8 | 52 | 6.86 | |
| Postcentral Gyrus | Right | 50 | −6 | 24 | 8.19 | |
| Postcentral Gyrus | Left | −56 | −10 | 24 | 7.88 | |
| Superior Temporal Gyrus | Right | 68 | −10 | 2 | 7.86 | |
| Superior Temporal Gyrus | Left | −66 | −26 | 2 | 6.52 | |
| Supramarginal Gyrus | Right | 64 | −36 | 16 | 6.86 | |
| Supramarginal Gyrus | Left | −58 | −42 | 16 | 6.71 | |
| Middle Temporal Gyrus | Right | 54 | −50 | 2 | 2.68 | |
| Middle Temporal Gyrus | Left | −52 | −50 | 2 | 2.85 | |
| Inferior Temporal Gyrus | Right | 54 | −52 | −10 | 7.44 | |
| Inferior Temporal Gyrus | Left | −48 | −54 | −10 | 9.97 | |
| Lateral Occipital Cortex | Left | −40 | −74 | −4 | 10.12 | |
| Lateral Occipital Cortex | Right | 50 | −76 | −4 | 11.31 | |
| Older Adults | ||||||
| Frontal Pole | Left | 154589 | −36 | 40 | 18 | 4.7 |
| Frontal Pole | Right | 46 | 38 | 18 | 5.62 | |
| Inferior Frontal Gyrus | Right | 46 | 34 | 12 | 6.32 | |
| Inferior Frontal Gyrus | Left | −46 | 32 | 12 | 5.92 | |
| Anterior Cingulate Gyrus | Left | −10 | 28 | 20 | 2.7 | |
| Anterior Cingulate Gyrus | Right | 6 | 24 | 22 | 5.61 | |
| Middle Frontal Gyrus | Right | 44 | 22 | 24 | 6.88 | |
| Middle Frontal Gyrus | Left | −46 | 14 | 38 | 4.94 | |
| Precentral Gyrus | Left | −46 | −2 | 52 | 8.61 | |
| Precentral Gyrus | Right | 46 | −8 | 52 | 7.1 | |
| Postcentral Gyrus | Right | 50 | −6 | 24 | 7.55 | |
| Postcentral Gyrus | Left | −56 | −10 | 24 | 7.03 | |
| Superior Temporal Gyrus | Right | 68 | −10 | 2 | 6.29 | |
| Superior Temporal Gyrus | Left | −66 | −26 | 2 | 6.92 | |
| Supramarginal Gyrus | Right | 64 | −36 | 16 | 4.74 | |
| Supramarginal Gyrus | Left | −58 | −42 | 16 | 5.79 | |
| Middle Temporal Gyrus | Right | 54 | −50 | 2 | 5.5 | |
| Middle Temporal Gyrus | Left | −52 | −50 | 2 | 5.02 | |
| Inferior Temporal Gyrus | Right | 54 | −52 | −10 | 6.12 | |
| Inferior Temporal Gyrus | Left | −48 | −54 | −10 | 7.3 | |
| Lateral Occipital Cortex | Left | −40 | −74 | −4 | 5.68 | |
| Lateral Occipital Cortex | Right | 50 | −76 | −4 | 7.08 | |
| Younger > Older | ||||||
| Paracingulate Gyrus | Middle | 731 | 3.81 | 0 | 30 | 38 |
| Anterior Cingulate Gyrus | Left | 3.86 | −2 | 10 | 24 | |
| Anterior Cingulate Gyrus | Right | 4.07 | 2 | −14 | 28 | |
| Temporal Occipital Fusiform Cortex | Left | 3554 | 5.65 | −36 | −46 | −12 |
| Lateral Occipital Cortex | Left | 5.56 | −50 | −76 | −4 | |
| Temporal Occipital Fusiform Cortex | Right | 4.34 | 38 | −56 | −12 | |
| Lateral Occipital Cortex | Right | 1103 | 4.91 | 46 | −78 | −6 |
| Older > Younger | ||||||
| Frontal Pole | Left | 194 | −4 | 66 | −4 | 3.25 |
| Frontal Medial Cortex | Right | 6 | 52 | −10 | 3.89 | |
| Frontal Medial Cortex | Left | −12 | 52 | −8 | 3.48 | |
| Paracingulate Gyrus | Right | 114 | 4 | 44 | 20 | 4.04 |
| Paracingulate Gyrus | Left | 278 | −14 | 36 | 24 | 3.34 |
| Superior Frontal Gyrus | Left | −16 | 32 | 40 | 3.53 | |
| Superior Temporal Gyrus | Right | 31792 | 54 | 0 | −12 | 2.77 |
| Middle Temporal Gyrus | Right | 64 | −18 | −8 | 6.29 | |
| Supramarginal Gyrus | Right | 56 | −22 | 36 | 7.21 | |
| Superior Parietal Lobule | Right | 40 | −46 | 54 | 3.16 | |
| Precentral Gyrus | Right | 32 | −24 | 58 | 3.28 | |
| Precentral Gyrus | Left | −36 | −20 | 58 | 3.76 | |
| Postcentral Gyrus | Right | 52 | −24 | 44 | 6.86 | |
| Postcentral Gyrus | Left | −52 | −24 | 52 | 6.17 | |
| Superior Temporal Gyrus | Left | 585 | −52 | −4 | −12 | 3.88 |
| Superior Parietal Lobule | Left | −40 | −46 | 54 | 3.55 | |
| Supramarginal Gyrus | Left | −46 | −38 | 44 | 4.42 | |
| Middle Temporal Gyrus | Left | −64 | −54 | 4 | 4.08 | |
| Posterior Cingulate Gyrus | Left | 52 | −6 | −50 | 14 | 3.55 |
| Cuneal Cortex | Right | 327 | 6 | −84 | 32 | 3.2 |
| Occipital Pole | Right | 6 | −92 | 14 | 3.17 | |
We also analyzed the patterns of activation to Go trials across the Go Bias condition and the No-Go Bias condition (i.e., Bias conditions). Across both age groups, Go trials in the Bias conditions elicited very similar regions as the All Go condition (Figure 3E & 3F, Table 3). The results comparing the two age groups were also similar to the patterns observed in the All Go condition (Figure 3G & 3H, Table 3).
Table 3.
Basic patterns of activation to Go trials in the Bias conditions & Main effect of age group.
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Go Trials in the All Go Condition | ||||||
| Younger Adults | ||||||
| Frontal Pole | Left | 146372 | −36 | 40 | 18 | 5.2 |
| Frontal Pole | Right | 46 | 38 | 18 | 6.02 | |
| Inferior Frontal Gyrus | Right | 46 | 34 | 12 | 4.88 | |
| Inferior Frontal Gyrus | Left | −46 | 32 | 12 | 7.68 | |
| Anterior Cingulate Gyrus | Left | −8 | 28 | 20 | 5.32 | |
| Anterior Cingulate Gyrus | Right | 6 | 24 | 22 | 5.57 | |
| Middle Frontal Gyrus | Right | 44 | 22 | 24 | 6.51 | |
| Middle Frontal Gyrus | Left | −46 | 14 | 38 | 6.54 | |
| Precentral Gyrus | Left | −46 | −2 | 52 | 8.87 | |
| Precentral Gyrus | Right | 46 | −8 | 52 | 6.54 | |
| Postcentral Gyrus | Right | 50 | −6 | 24 | 9.15 | |
| Postcentral Gyrus | Left | −56 | −10 | 24 | 8.77 | |
| Superior Temporal Gyrus | Right | 68 | −10 | 2 | 7.99 | |
| Superior Temporal Gyrus | Left | −66 | −26 | 2 | 5.89 | |
| Supramarginal Gyrus | Right | 64 | −36 | 16 | 7.42 | |
| Supramarginal Gyrus | Left | −58 | −42 | 16 | 7.14 | |
| Middle Temporal Gyrus | Right | 54 | −50 | 2 | 4.27 | |
| Middle Temporal Gyrus | Left | −52 | −50 | 2 | 4.76 | |
| Inferior Temporal Gyrus | Right | 54 | −52 | −10 | 8.45 | |
| Inferior Temporal Gyrus | Left | −48 | −54 | −10 | 9.99 | |
| Lateral Occipital Cortex | Left | −40 | −74 | −4 | 12.94 | |
| Lateral Occipital Cortex | Right | 50 | −76 | −4 | 11.59 | |
| Older Adults | ||||||
| Frontal Pole | Left | 164209 | −36 | 40 | 18 | 6.17 |
| Frontal Pole | Right | 46 | 38 | 18 | 7.96 | |
| Inferior Frontal Gyrus | Right | 46 | 34 | 12 | 9.39 | |
| Inferior Frontal Gyrus | Left | −46 | 32 | 12 | 8.76 | |
| Anterior Cingulate Gyrus | Left | −8 | 28 | 20 | 2.72 | |
| Anterior Cingulate Gyrus | Right | 6 | 24 | 22 | 4.22 | |
| Middle Frontal Gyrus | Right | 44 | 22 | 24 | 8.81 | |
| Middle Frontal Gyrus | Left | −46 | 14 | 38 | 6.69 | |
| Precentral Gyrus | Left | −46 | −2 | 52 | 11.31 | |
| Precentral Gyrus | Right | 46 | −8 | 52 | 8.85 | |
| Postcentral Gyrus | Right | 50 | −6 | 24 | 10.31 | |
| Postcentral Gyrus | Left | −56 | −10 | 24 | 10.09 | |
| Superior Temporal Gyrus | Right | 68 | −10 | 2 | 9.41 | |
| Superior Temporal Gyrus | Left | −66 | −26 | 2 | 10.06 | |
| Supramarginal Gyrus | Right | 64 | −36 | 16 | 8.12 | |
| Supramarginal Gyrus | Left | −58 | −42 | 16 | 8.33 | |
| Middle Temporal Gyrus | Right | 54 | −50 | 2 | 7.36 | |
| Middle Temporal Gyrus | Left | −52 | −50 | 2 | 7.58 | |
| Inferior Temporal Gyrus | Right | 54 | −52 | −10 | 8.35 | |
| Inferior Temporal Gyrus | Left | −48 | −54 | −10 | 10.48 | |
| Lateral Occipital Cortex | Left | −40 | −74 | −4 | 7.91 | |
| Lateral Occipital Cortex | Right | 50 | −76 | −4 | 10.29 | |
| Younger > Older | ||||||
| Anterior Cingulate Gyrus | Left | 1204 | −4 | 18 | 30 | 4.85 |
| Anterior Cingulate Gyrus | Right | 4 | −8 | 28 | 7.52 | |
| Parahippocampal Gyrus | Left | 16499 | −10 | −4 | −22 | 4.4 |
| Lateral Occipital Cortex | Left | −40 | −72 | −2 | 3.23 | |
| Lateral Occipital Cortex | Right | 30 | −88 | −2 | 5.36 | |
| Older > Younger | ||||||
| Frontal Pole | Right | 13 | 22 | 62 | −4 | 3.55 |
| Frontal Pole | Left | 200 | −26 | 58 | 2 | 3.69 |
| Frontal Medial Cortex | Right | 38 | 6 | 36 | −20 | 3.11 |
| Subcallosal Cortex | Right | 6 | 30 | −16 | 3.88 | |
| Middle Frontal Gyrus | Right | 325 | 28 | 34 | 32 | 4.52 |
| Superior Frontal Gyrus | Right | 32205 | 24 | 26 | 60 | 7.2 |
| Middle Frontal Gyrus | Left | −34 | 2 | 66 | 7.5 | |
| Supramarginal Gyrus | Right | 58 | −20 | 30 | 7.42 | |
| Postcentral Gyrus | Left | −44 | −28 | 40 | 7.22 | |
| Middle Temporal Gyrus | Right | 60 | −32 | 0 | 7.32 | |
| Superior Parietal Lobule | Left | −36 | −48 | 64 | 8.06 | |
| Middle Temporal Gyrus | Left | 3933 | −56 | 2 | −18 | 5.96 |
| Superior Temporal Gyrus | Left | −66 | −14 | 4 | 6.14 | |
| Angular Gyrus | Left | 31 | −38 | −60 | 20 | 3.21 |
| Lingual Gyrus | Right | 1605 | 16 | −66 | −4 | 5.49 |
| Lingual Gyrus | Left | −8 | −78 | −4 | 5.6 | |
3.2.1.2. Effects of Naming Difficulty
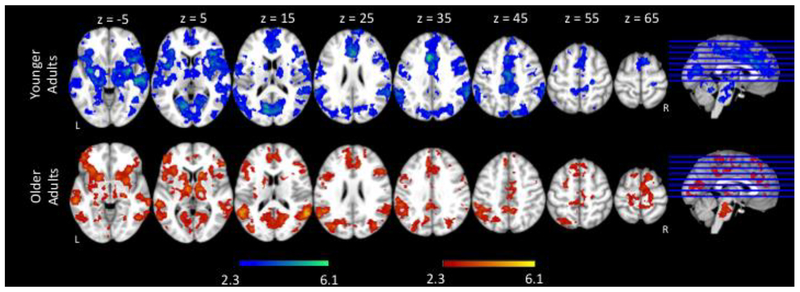
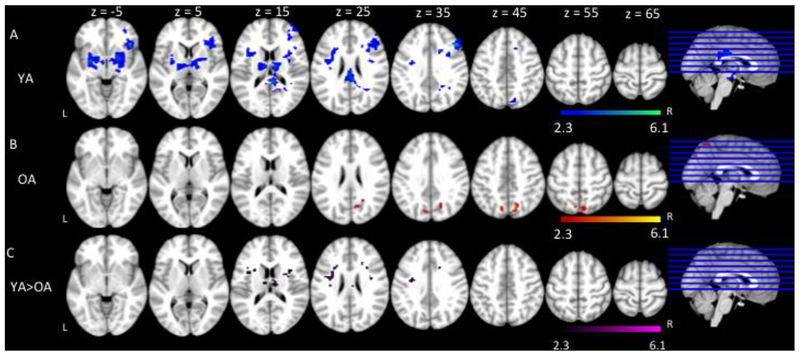
To investigate the neural response to increases in language production demands, we compared the fMRI activation of Go trials between the two Bias conditions (Go Bias < No-Go Bias) for all participants and then examined the results for age group differences. Both older and younger adults showed significant increases in activation as naming difficulty increased in bilateral inferior frontal gyri extending to bilateral frontal pole; bilateral anterior cingulate gyri; bilateral pre- and post- central gyri; bilateral supramarginal gyri extending to bilateral angular gyri; and bilateral occipital cortex (Figure 4, Table 4).
Figure 4.
fMRI activation as a function of naming difficulty (Go trials: Go Bias < No-Go Bias) for younger adults (YA) and older adults (OA). Slices are depicted in increments of 10, starting at z = −5 and ending at z = 65.
Table 4.
Naming difficulty by age group (Go Bias < No-Go Bias)
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Younger Adults | ||||||
| Frontal Pole | Left | 11666 | −6 | 60 | 16 | 2.99 |
| Frontal Pole | Right | 10 | 52 | 42 | 3.59 | |
| Paracingulate Gyrus | Right | 10 | 46 | 8 | 5.44 | |
| Anterior Cingulate Gyrus | Right | 2 | 28 | 24 | 5.8 | |
| Anterior Cingulate Gyrus | Left | −4 | 22 | 30 | 6.3 | |
| Superior Frontal Gyrus | Left | −14 | 22 | 62 | 3.13 | |
| Superior Frontal Gyrus | Right | 10 | 22 | 62 | 3.73 | |
| Posterior Cingulate Gyrus | Left | −8 | −26 | 42 | 2.84 | |
| Posterior Cingulate Gyrus | Right | 2 | −28 | 42 | 2.93 | |
| Precuneus Cortex | Right | 4 | −42 | 48 | 2.92 | |
| Inferior Frontal Gyrus | Right | 49660 | 46 | 34 | 14 | 2.57 |
| Inferior Frontal Gyrus | Left | −48 | 30 | 8 | 2.7 | |
| Frontal Orbital Cortex | Left | −34 | 28 | −4 | 3.65 | |
| Frontal Operculum Cortex | Right | 36 | 22 | 8 | 3.8 | |
| Frontal Operculum Cortex | Left | −36 | 16 | 8 | 3.24 | |
| Middle Frontal Gyrus | Right | 44 | 14 | 36 | 2.93 | |
| Insular Cortex | Right | 42 | 14 | −8 | 4.28 | |
| Insular Cortex | Left | −36 | 12 | −8 | 2.82 | |
| Central Opercular Cortex | Right | 48 | 4 | 2 | 4.6 | |
| Amygdala | Right | 28 | −2 | −14 | 5.84 | |
| Precentral Gyrus | Right | 52 | −4 | 40 | 2.71 | |
| Precentral Gyrus | Left | −42 | −6 | 48 | 3.3 | |
| Central Opercular Cortex | Right | 42 | −10 | 14 | 2.67 | |
| Superior Temporal Gyrus | Right | 52 | −16 | −6 | 3.39 | |
| Postcentral Gyrus | Right | 44 | −18 | 42 | 2.68 | |
| Postcentral Gyrus | Left | −44 | −18 | 40 | 2.51 | |
| Middle Temporal Gyrus | Right | 54 | −22 | −6 | 6.37 | |
| Superior Temporal Gyrus | Right | 60 | −24 | 0 | 3.58 | |
| Supramarginal Gyrus | Right | 58 | −38 | 44 | 3.76 | |
| Supramarginal Gyrus | Left | −58 | −46 | 32 | 5.89 | |
| Angular Gyrus | Right | 58 | −52 | 40 | 3.47 | |
| Angular Gyrus | Left | −52 | −58 | 30 | 2.49 | |
| Intracalcarine Cortex | Left | −12 | −66 | 8 | 4.54 | |
| Intracalcarine Cortex | Left | −12 | −78 | 12 | 5.75 | |
| Lateral Occipital Cortex | Left | −18 | −82 | 48 | 2.92 | |
| Older Adults | ||||||
| Frontal Pole | Right | 43142 | 18 | 56 | 30 | 2.88 |
| Paracingulate Gyrus | Left | −6 | 46 | 20 | 3.11 | |
| Paracingulate Gyrus | Right | 8 | 42 | 20 | 2.95 | |
| Superior Frontal Gyrus | Right | 4 | 32 | 54 | 3.02 | |
| Frontal Orbital Cortex | Left | −30 | 30 | 2 | 3.69 | |
| Anterior Cingulate Gyrus | Left | −4 | 26 | 30 | 3.05 | |
| Insular Cortex | Right | 32 | 24 | −4 | 3.85 | |
| Middle Frontal Gyrus | Right | 50 | 22 | 30 | 3.47 | |
| Anterior Cingulate Gyrus | Right | 8 | 22 | 30 | 2.98 | |
| Frontal Operculum Cortex | Left | −32 | 20 | 10 | 3.7 | |
| Inferior Frontal Gyrus | Right | 54 | 18 | 10 | 2.85 | |
| Inferior Frontal Gyrus | Left | −50 | 16 | 24 | 2.91 | |
| Precentral Gyrus | Right | 52 | 6 | 38 | 2.73 | |
| Precentral Gyrus | Left | −22 | −22 | 64 | 2.74 | |
| Middle Temporal Gyrus | Right | 56 | −12 | −12 | 2.71 | |
| Superior Temporal Gyrus | Right | 56 | −30 | 0 | 2.73 | |
| Postcentral Gyrus | Left | −22 | −34 | 66 | 2.99 | |
| Postcentral Gyrus | Right | 22 | −36 | 62 | 2.62 | |
| Supramarginal Gyrus | Right | 60 | −42 | 30 | 2.5 | |
| Supramarginal Gyrus | Left | −60 | −44 | 30 | 3.19 | |
| Angular Gyrus | Right | 60 | −50 | 18 | 6.01 | |
| Angular Gyrus | Left | −50 | −54 | 38 | 3.72 | |
| Intracalcarine Cortex | Right | 14 | −66 | 12 | 3.98 | |
We also compared the two age groups in terms of the increases in activation as naming difficulty increased (from the Go Bias condition to the No-Go Bias condition). However, the patterns of activation did not differ between groups.
3.2.1.3. Relations Between Reaction Time and fMRI activation
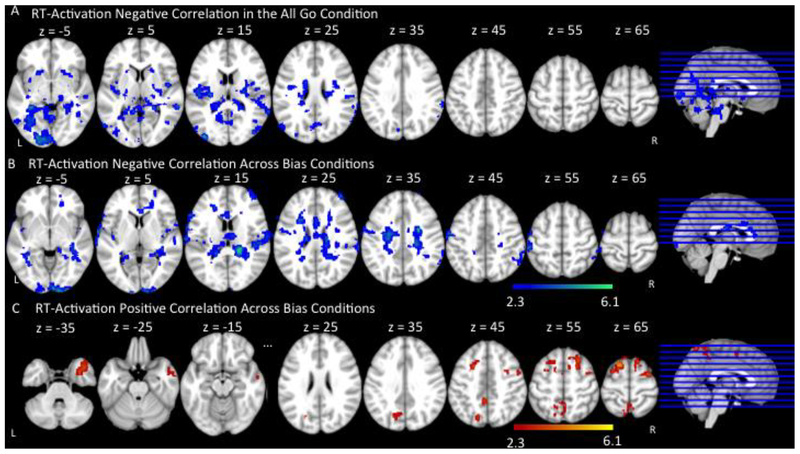
To understand the relationship between older adults’ brain activation and behavioral performance, we correlated older adults’ fMRI activation with their naming reaction times to Go trials in the All Go condition and across both Bias conditions. In the All Go condition, Negative correlations (e.g., faster reaction times associated with greater activation) were found in right supramarginal gyrus extending to angular gyrus and middle temporal gyrus, and bilateral occipital cortex (Figure 5A, Table 5). There were no significant positive correlations (i.e., longer reaction times associated with greater brain activation) between older adults’ naming times and brain activation across all trials in the All Go condition.
Figure 5.
Correlation between reaction time and fMRI activation across Go trials in older adults. (A) represents regions in which faster reaction time was associated with more activation (Negative Correlation) in the All Go Condition. (B) represents regions in which faster reaction time was associated with more activation (Negative Correlation) across Bias Conditions. (C) represents regions in which longer reaction time was associated with more activation (Positive Correlation) across Bias Conditions. Slices are depicted in increments of 10, starting at z = −5 and ending at z = 65.
Table 5.
Correlation between reaction time and fMRI activation across Go trials in the All Go Condition and the Bias Conditions in older adults.
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Negative Correlations in All Go Condition | ||||||
| Central Opercular Cortex | Right | 16706 | 42 | −4 | 10 | 5.31 |
| Middle Temporal Gyrus | Right | 62 | −38 | 4 | 3.6 | |
| Supramarginal Gyrus | Right | 54 | −42 | 20 | 2.32 | |
| Angular Gyrus | Right | 64 | −50 | 20 | 2.87 | |
| Lateral Occipital Cortex | Right | 60 | −80 | 12 | 3.12 | |
| Lateral Occipital Cortex | Left | −36 | −90 | 16 | 5.14 | |
| Occipital Pole | Right | 8 | −100 | −8 | 5.17 | |
| No significant positive correlations | ||||||
| Negative Correlations in Bias Conditions | ||||||
| Frontal Pole | Right | 10949 | 44 | 48 | 18 | 2.68 |
| Anterior Cingulate Gyrus | Right | 2 | 20 | 18 | 2.76 | |
| Anterior Cingulate Gyrus | Left | −2 | 20 | 18 | 2.88 | |
| Precentral Gyrus | Left | −58 | −4 | 38 | 2.66 | |
| Postcentral Gyrus | Right | 66 | −14 | 18 | 3.2 | |
| Postcentral Gyrus | Left | −50 | −32 | 60 | 4.97 | |
| Supramarginal Gyrus | Right | 60 | −44 | 46 | 2.7 | |
| Angular Gyrus | Right | 58 | −50 | 44 | 2.72 | |
| Occipital Pole | Right | 919 | 16 | −102 | −8 | 4.72 |
| Occipital Pole | Left | −14 | −104 | −2 | 4.13 | |
| Positive Correlations in Bias Conditions | ||||||
| Superior Frontal Gyrus | Right | 1500 | 26 | 22 | 58 | 4.18 |
| Superior Frontal Gyrus | Left | −14 | 14 | 66 | 5.06 | |
| Middle Frontal Gyrus | Right | 32 | 0 | 64 | 4.01 | |
| Temporal Pole | Right | 586 | 40 | 16 | −40 | 4.83 |
| Precuneus Cortex | Left | 785 | −4 | −46 | 44 | 3.66 |
| Precuneus Cortex | Right | 2 | −52 | 58 | 2.71 | |
| Lateral Occipital Cortex | Left | −18 | −74 | 50 | 4.27 | |
Across the Bias conditions, negative correlations were found in bilateral anterior cingulate gyri, left pre-central gyrus, bilateral post-central gyri extending to right supramarginal gyrus and angular gyrus (Figure 5B, Table 5). These patterns are consistent with older adults’ brain-behavior correlations in the All Go condition, although more spatially extensive. Significant positive correlations between older adults’ naming times and brain activation across Go trials in the Bias conditions were found in bilateral superior frontal gyri extending to right middle frontal gyrus, right temporal pole, and bilateral precuneus cortex extending to left occipital cortex (Figure 5C, Table 5).
We were also interested in whether these brain-behavior correlations in older adults differed as a function of naming difficulty. That is, if the brain-behavior correlations represent compensation or dedifferentiation, would naming difficulty affect this pattern? To investigate this question, we compared both positive and negative correlations between older adults’ brain activation and reaction times, across the two Bias conditions. However, there were no differences between the positive or the negative correlations comparing the Bias conditions.
3.2.1.4. Effects of General Task Demands in Naming — Secondary Analyses
To explore the effect of general task demands on participants’ brain activation, secondary analyses were conducted comparing the fMRI activation of Go trials between the All Go and the Bias conditions (All Go < Bias Conditions) within and between age groups. Younger adults showed significant increases in activation as general task demands increased in right inferior and middle frontal gyri extending to right frontal pole, bilateral posterior cingulate gyri, right angular gyrus extending to occipital cortex (Figure 6A, Table 6). Older adults showed significant increases in activation with increased task demands in bilateral precuneus cortex and right lateral occipital cortex (Figure 6B, Table 6). Comparing the increases in activation between groups, younger adults showed larger increases in activation in right middle frontal gyrus and bilateral posterior cingulate gyrus compared to older adults (Figure 6C, Table 6). There were no regions in which older adults elicited greater activation than younger adults.
Figure 6.
fMRI activation as a function of general task demands (Go trials: All Go < Bias conditions) for A) Younger Adults (YA), B) Older Adults (OA), and C) Younger Adults > Older Adults. Colored regions represent areas of statistically significant activation. Slices are depicted in increments of 10, starting at z = −5 and ending at z = 65.
Table 6.
Task demands on Go trials by age group (All Go < Bias Conditions)
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Younger Adults | ||||||
| Frontal Pole | Right | 1901 | 44 | 50 | 18 | 2.54 |
| Inferior Frontal Gyrus | Right | 1901 | 44 | 28 | 4 | 2.44 |
| Middle Frontal Gyrus | Right | 46 | 26 | 36 | 5.21 | |
| Frontal Orbital Cortex | Right | 42 | 26 | −4 | 5.14 | |
| Parahippocampal Gyrus | Right | 538 | 26 | 0 | −34 | 3.89 |
| Temporal Pole | Right | 24 | 4 | −42 | 3.74 | |
| Posterior Cingulate Gyrus | Left | 3841 | −6 | −20 | 28 | 4.76 |
| Posterior Cingulate Gyrus | Right | 6 | −22 | 28 | 4.71 | |
| Angular Gyrus | Right | 73 | 36 | −52 | 36 | 2.78 |
| Supracalcarine Cortex | Right | 24 | −62 | 20 | 2.58 | |
| Lateral Occipital Cortex | Right | 60 | 16 | −74 | 48 | 3.62 |
| Precuneus Cortex | Right | 6 | −76 | 46 | 3.02 | |
| Older Adults | ||||||
| Lateral Occipital Cortex | Right | 370 | 3.99 | 10 | −72 | 58 |
| Precuneus Cortex | Right | 3.92 | 8 | −66 | 56 | |
| Precuneus Cortex | Left | 68 | 2.87 | −10 | −70 | 44 |
| Younger > Older | ||||||
| Inferior Frontal Gyrus | Right | 201 | 32 | 12 | 24 | 2.93 |
| Posterior Cingulate Gyrus | Right | 58 | 8 | −16 | 26 | 3.66 |
| Posterior Cingulate Gyrus | Left | 13 | −8 | −18 | 28 | 2.71 |
To investigate the relationship between reaction time and brain activation and how this changed with general task demands, we compared older adults’ positive and negative correlations in the All Go and the Bias conditions. The negative RT-activation correlation in the All Go condition was stronger compared to the RT-activation correlation in the Bias conditions for older adults, in bilateral occipital cortex extending to bilateral precuneus cortex, and in right insular cortex. There were no differences in the strength of the positive correlations.
3.2.2. No-Go Trials: Effects of Age and Task Difficulty on Cognitive Control
3.2.2.1. Basic Pattern of Activation and Main Effect of Age
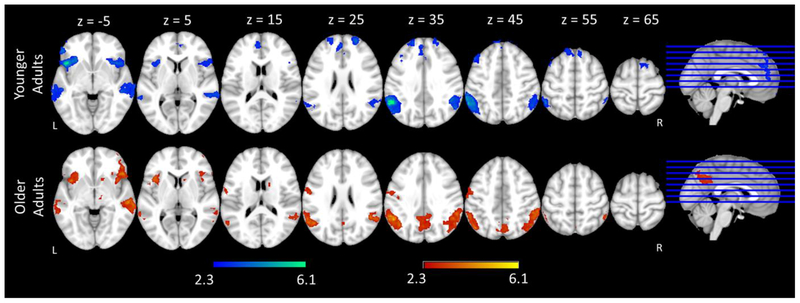
No-Go trials, across participants and conditions, elicited activation in typical regions related to cognitive control (e.g., response inhibition, attention, conflict monitoring, etc.) such as bilateral frontal pole, bilateral superior, middle and inferior frontal gyri, and bilateral paracingulate gyri. No-Go trials also activated other regions such as bilateral precentral gyri and bilateral occipital cortex (Figure 7A & 7B & Table 7). Statistical comparisons between groups showed that younger adults elicited greater activation than older adults in bilateral occipital cortex (Figure 7C, Table 7). Older adults had more extensive activation in bilateral frontal pole extending to right paracingulate gyrus and right anterior cingulate gyrus, left orbital-frontal cortex, bilateral middle and inferior frontal gyri, bilateral pre- and post- central gyri, bilateral supramarginal gyri extending to right lingual gyrus, right posterior cingulate gyrus, and bilateral occipital cortex (Figure 7D, Table 7).
Figure 7.
Basic patterns of activation and main effect of age for No-Go trials. Shown is an overview of the regions in which there was significant activation in (A) Younger Adults, (B) Older Adults, (C) Younger Adults > Older Adults, (D) Older Adults > Younger Adults.
Table 7 –
Basic patterns of activation to No-Go trials & Main effect of age group.
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| No-Go Trials Across Conditions | ||||||
| Younger Adults | ||||||
| Frontal Pole | Left | 72709 | −52 | 36 | 18 | 6.37 |
| Frontal Pole | Right | 52 | 36 | 18 | 5.92 | |
| Middle Frontal Gyrus | Left | −40 | 32 | 22 | 8 | |
| Middle Frontal Gyrus | Right | 40 | 32 | 22 | 6.62 | |
| Inferior Frontal Gyrus | Left | −52 | 32 | 20 | 7.64 | |
| Inferior Frontal Gyrus | Right | 52 | 32 | 20 | 5.9 | |
| Superior Frontal Gyrus | Right | 6 | 20 | 64 | 4.5 | |
| Superior Frontal Gyrus | Left | −12 | 12 | 64 | 4.84 | |
| Paracingulate Gyrus | Left | −6 | 26 | 38 | 8.26 | |
| Paracingulate Gyrus | Right | 10 | 24 | 38 | 6.93 | |
| Precentral Gyrus | Right | 44 | 6 | 30 | 7.98 | |
| Precentral Gyrus | Left | −54 | 6 | 30 | 5.82 | |
| Temporal Occipital Cortex | Right | 38 | −54 | −16 | 13.34 | |
| Inferior Lateral Occipital Cortex | Right | 40 | −78 | −10 | 13.68 | |
| Inferior Lateral Occipital Cortex | Left | −42 | −86 | 0 | 13.88 | |
| Older Adults | ||||||
| Frontal Pole | Right | 107838 | 34 | 56 | 20 | 5.61 |
| Frontal Pole | Left | −36 | 54 | 10 | 5.16 | |
| Paracingulate Gyrus | Left | −4 | 32 | 30 | 5.22 | |
| Paracingulate Gyrus | Right | 8 | 28 | 30 | 6.98 | |
| Superior Frontal Gyrus | Right | 6 | 30 | 48 | 7.61 | |
| Superior Frontal Gyrus | Left | −12 | 10 | 62 | 6.97 | |
| Inferior Frontal Gyrus | Right | 46 | 12 | 24 | 7.99 | |
| Inferior Frontal Gyrus | Left | −46 | 10 | 24 | 8.38 | |
| Precentral Gyrus | Left | −58 | 4 | 30 | 6.34 | |
| Precentral Gyrus | Right | 52 | 4 | 30 | 8.27 | |
| Middle Frontal Gyrus | Right | 36 | 2 | 54 | 9.4 | |
| Middle Frontal Gyrus | Left | −38 | 0 | 54 | 9.28 | |
| Postcentral Gyrus | Right | 46 | −32 | 54 | 5.38 | |
| Postcentral Gyrus | Left | −46 | −32 | 54 | 4.4 | |
| Supramarginal Gyrus | Right | 46 | −38 | 54 | 7.49 | |
| Supramarginal Gyrus | Left | −50 | −44 | 54 | 6.45 | |
| Superior Parietal Lobule | Right | 34 | −44 | 54 | 7.26 | |
| Superior Parietal Lobule | Left | −38 | −46 | 54 | 9.14 | |
| Inferior Temporal Gyrus | Left | −42 | −50 | −14 | 15.4 | |
| Temporal Occipital Cortex | Right | 44 | −56 | −12 | 12.66 | |
| Temporal Occipital Cortex | Left | −44 | −58 | −14 | 18.5 | |
| Occipital Fusiform Gyrus | Right | 36 | −68 | −14 | 19.8 | |
| Lateral Occipital Cortex | Left | −36 | −74 | 4 | 6.55 | |
| Lateral Occipital Cortex | Right | 34 | −82 | 4 | 9.93 | |
| Occipital Pole | Left | −24 | −92 | −8 | 15.6 | |
| Younger > Older | ||||||
| Temporal Occipital Cortex | Right | 1698 | 34 | −50 | −12 | 5.38 |
| Temporal Occipital Cortex | Left | 3512 | −38 | −48 | −12 | 7.21 |
| Occipital Fusiform Gyrus | Left | −34 | −66 | −2 | 5.98 | |
| Lateral Occipital Cortex | Left | −48 | −76 | −4 | 5.43 | |
| Lateral Occipital Cortex | Right | 42 | −80 | −6 | 5.13 | |
| Older > Younger | ||||||
| Frontal Pole | Right | 57 | 12 | 64 | −12 | 4.7 |
| Frontal Pole | Left | 51 | −32 | 48 | 32 | 3.71 |
| Paracingulate Gyrus | Right | 76 | 6 | 34 | 30 | 2.76 |
| Anterior Cingulate Gyrus | Right | 8 | 30 | 22 | 2.84 | |
| Frontal Orbital Cortex | Left | 455 | −20 | 26 | −22 | 3.64 |
| Inferior Frontal Gyrus | Left | −52 | 24 | −6 | 3.77 | |
| Temporal Pole | Left | −58 | 12 | −12 | 4.09 | |
| Middle Frontal Gyrus | Right | 76 | 44 | 24 | 46 | 4.89 |
| Inferior Frontal Gyrus | Right | 36699 | 58 | 10 | 8 | 5.08 |
| Middle Frontal Gyrus | Left | −36 | 2 | 62 | 7.44 | |
| Precentral Gyrus | Right | 58 | 6 | 38 | 4.08 | |
| Precentral Gyrus | Left | −46 | −8 | 56 | 4.58 | |
| Postcentral Gyrus | Right | 58 | −18 | 30 | 7.07 | |
| Postcentral Gyrus | Left | −56 | −28 | 48 | 3.62 | |
| Supramarginal Gyrus | Right | 50 | −28 | 40 | 6.81 | |
| Supramarginal Gyrus | Left | −42 | −48 | 48 | 3.94 | |
| Superior Parietal Lobule | Left | −38 | −50 | 66 | 6.82 | |
| Lateral Occipital Cortex | Right | 8 | −64 | 64 | 7.47 | |
| Lateral Occipital Cortex | Left | −10 | −74 | 54 | 4.82 | |
| Posterior Cingulate Gyrus | Right | 41 | 6 | −36 | 36 | 3.25 |
| Lingual Gyrus | Right | 54 | 16 | −64 | −10 | 2.38 |
3.2.2.2. Effects of Inhibition Difficulty
To examine the role of response inhibition difficulty on neural activity, we compared activation to No-Go trials as inhibition demands increased (No-Go Bias < Go Bias) for each group. Across both age groups, the Go Bias condition elicited more activation than the No-Go Bias condition in right inferior frontal gyri/insular cortex, right middle temporal gyrus, bilateral supramarginal gyri extending to left angular gyri (See Figure 8 and Table 8). Although there were group differences in activation to No-Go trials overall, there was no significant interaction between Condition and Age group (i.e., no significant group differences in the increases in activation across the two conditions, No-Go Bias < Go Bias).
Figure 8.
fMRI activation as a function of inhibition difficulty (No-Go trials: No-Go Bias< Go Bias) for younger adults (YA) and older adults (OA). Slices are depicted in increments of 10, starting at z = −5 and ending at z = 65.
Table 8.
Effect of inhibition difficulty (No-Go Bias < Go Bias)
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Younger Adults | ||||||
| Frontal Pole | Right | 2793 | 24 | 54 | 22 | 4.18 |
| Paracingulate Gyrus | Left | −8 | 42 | 22 | 3.83 | |
| Superior Frontal Gyrus | Right | 10 | 34 | 58 | 4.23 | |
| Middle Frontal Gyrus | Left | 412 | −40 | 26 | 50 | 4.54 |
| Frontal Orbital Cortex | Left | 1960 | −30 | 20 | −16 | 5.32 |
| Frontal Orbital Cortex | Right | 1102 | 38 | 20 | −14 | 3.81 |
| Insular Cortex | Right | 42 | 18 | −4 | 4.4 | |
| Inferior Frontal Gyrus | Right | 52 | 18 | 10 | 3.8 | |
| Frontal Operculum Cortex | Right | 48 | 18 | 0 | 3.64 | |
| Superior Temporal Gyrus | Left | 1197 | −64 | −20 | −6 | 4.04 |
| Middle Temporal Gyrus | Left | −64 | −32 | −8 | 4.05 | |
| Superior Temporal Gyrus | Right | 1249 | 62 | −26 | −2 | 3.95 |
| Middle Temporal Gyrus | Right | 70 | −34 | 0 | 4.15 | |
| Supramarginal Gyrus | Right | 1328 | 54 | −40 | 40 | 4.09 |
| Angular Gyrus | Right | 58 | −58 | 28 | 4.23 | |
| Supramarginal Gyrus | Left | 2237 | −50 | −46 | 44 | 5.07 |
| Angular Gyrus | Left | −56 | −52 | 42 | 7.8 | |
| Older Adults | ||||||
| Frontal Pole | Right | 1139 | 48 | 38 | −4 | 4.11 |
| Frontal Orbital Cortex | Right | 32 | 24 | −10 | 5.55 | |
| Insular Cortex | Right | 38 | 14 | −8 | 4.26 | |
| Frontal Operculum Cortex | Right | 44 | 14 | 0 | 3.45 | |
| Frontal Operculum Cortex | Left | 1019 | −36 | 16 | 8 | 4.4 |
| Insular Cortex | Left | −40 | 14 | −6 | 4.38 | |
| Putamen | Right | 565 | 20 | 14 | −8 | 3.31 |
| Amygdala | Right | 22 | −2 | −14 | 3.35 | |
| Temporal Pole | Right | 542 | 52 | 8 | −24 | 3.69 |
| Precentral Gyrus | Left | 670 | −50 | −6 | 44 | 3.87 |
| Postcentral Gyrus | Left | −56 | −10 | 34 | 3.57 | |
| Middle Temporal Gyrus | Right | 3641 | 68 | −18 | −12 | 5.08 |
| Supramarginal Gyrus | Right | 58 | −44 | 30 | 4.79 | |
| Supramarginal Gyrus | Left | 2856 | −56 | −48 | 36 | 4.84 |
| Angular Gyrus | Left | −52 | −54 | 36 | 5.7 | |
| Lateral Occipital Cortex | Left | −46 | −68 | 44 | 4.91 | |
| Precuneus Cortex | Left | 1292 | −2 | −62 | 40 | 3.82 |
| Precuneus Cortex | Right | 4 | −62 | 40 | 3.58 | |
3.2.2.3. Relations Between Activation and Commission Error Rates
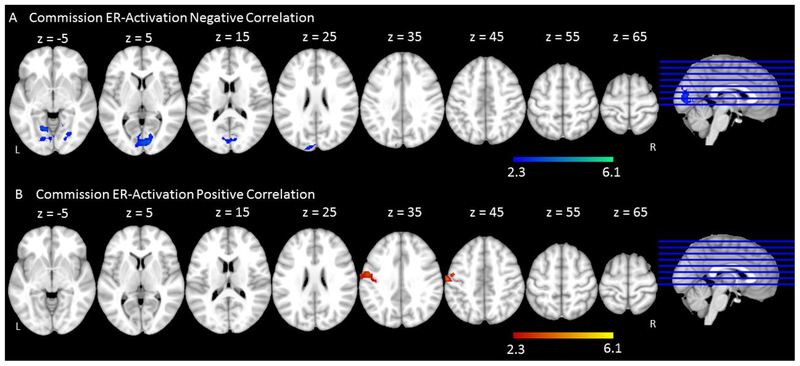
As with the Go trials, we were interested in understanding the brain-behavior relationships for No-Go trials. The commission error rate was used as the index of behavioral performance for No-Go trials as it represents an index of accurate response inhibition. Following the same logic as our analyses with Go trials, we correlated older adults’ brain activation with their commission error rate across all No-Go trials. Negative correlations between commission error rates and brain activation were found in bilateral lingual gyri extending to bilateral intra-calcarine cortex (Figure 9A & Table 9). Positive correlations between commission error rates and older adults’ brain activation were reflected in left pre- and post-central gyri (Figure 9B & Table 9).
Figure 9.
Correlations between commission error rate and fMRI activation across No-Go trials in older adults. (A) Negative correlation – Regions in which lower commission error rate was associated with more activation. (B) Positive correlation – Regions in which higher commission error rate was associated with more activation.
Table 9.
Correlation between commission error rate and fMRI activation across No-Go trials in older adults
| Hemisphere | Voxels | Coordinates (mm) | Z value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Negative Correlations | ||||||
| Lingual Gyrus | Left | 1818 | −12 | −62 | −6 | 3.76 |
| Lingual Gyrus | Right | 4 | −76 | 0 | 3.52 | |
| Intracalcarine Cortex | Right | 12 | −84 | 4 | 4.09 | |
| Intracalcarine Cortex | Left | −4 | −86 | 6 | 3.27 | |
| Positive Correlations | ||||||
| Precentral Gyrus | Left | 586 | −50 | −8 | 46 | 3.74 |
| Postcentral Gyrus | Left | −64 | −8 | 32 | 3.03 | |
We also examined whether the strength of these cortical activation-behavior correlations differed as a function of condition for the older adults. The results indicated that the negative correlations in the Go Bias and No-Go Bias conditions were not significantly different from each other. However, for the positive cortical activation-behavior correlations, correlations in the No-Go Bias condition were stronger than the correlations in the Go Bias condition in left middle and inferior frontal gyri extending to left frontal pole, bilateral pre- and post- central gyri, bilateral thalamus, and bilateral precuneus cortex.
4. Discussion
This study examined behavioral and neural aspects of language production and response inhibition in older and younger adults using a phonological Go/No-Go picture naming paradigm. Proportions of Go (overt naming) and No-Go (withhold naming) trials were varied to manipulate task difficulty for both naming pictures and inhibiting responses. Although other studies have examined the role of task difficulty on cognition, this study is the first to specifically investigate the effect of task difficulty and brain-behavior correlations in older adults in the domain of language production. We hypothesized that participants’ behavioral performance, fMRI activation, and their relationships would change as a function of both age group and task difficulty. In general, the results are partially consistent with our predictions based on the CRUNCH model (Reuter-Lorenz & Cappell, 2008).
Consistent with previous studies, we found significant age differences for both Go trials and No-Go trials. Behaviorally, older adults exhibited slower reaction times and more omission errors on Go trials and more commission errors on No-Go trials than younger adults, consistent with age-related declines in both language production and response inhibition. In terms of functional activation, for both Go trials and No-Go trials, older adults showed greater activation than younger adults in many regions including typical language regions, their right hemisphere homologues, and frontal regions. These patterns are consistent with previous studies that have observed more bilateral and frontal activations in older adults (Cabeza, 2002; Davis et al., 2008; Destrieux et al., 2012; Diaz et al., 2014; Diaz et al., In Press; Diaz et al., 2016; Meinzer et al., 2009; Meinzer et al., 2012; Persson et al., 2004; Wierenga et al., 2008). Younger adults, on the other hand, elicited greater activation than older adults when naming pictures in bilateral anterior cingulate gyri and occipital cortex, suggesting greater engagement of cognitive control and primary visual processing regions, consistent with previous work from our lab (e.g., Diaz et al., 2014) as well as others (Davis et al., 2008).
We also predicted that increases in naming difficulty would elicit declines in behavioral performance and increased activation for both age groups, which we observed. Behaviorally, all participants’ reaction times for Go trials increased as naming demands increased (Go Bias < No-Go Bias). Additionally, as naming demands increased, participants elicited greater activation bilaterally in several frontal and temporal regions, as well as in supramarginal and angular gyri. While the left hemisphere component of these regions comprise a core language network (Geranmayeh et al., 2012; Geranmayeh, Wise, Mehta, & Leech, 2014; Hickok & Poeppel, 2007; Indefrey & Levelt, 2004; Poldrack et al., 2001; Poldrack et al., 1999), the increased activation in the right homologues suggests the recruitment of additional resources in response to increased naming difficulty for all adults, consistent with previous observations (e.g., Bench et al., 1993; de Zubicaray, Wilson, McMahon, & Muthiah, 2001). The increased involvement of right insula when there were fewer Go trials (i.e., No-Go Bias condition) may also reflect higher attentional demands of infrequent trials (Wijeakumar et al., 2015).
Similarly, for the No-Go trials, there were more commission errors and increased fMRI activation in the Go Bias condition compared to the No-Go Bias condition, consistent with the intended manipulation of response inhibition difficulty. Response inhibition is multifaceted, involving several different cognitive functions including response control, attentional monitoring, and proactive and reactive control. The increased activation in frontal regions (such as bilateral superior and middle frontal gyri) in response to increased inhibitory demands is consistent with previous studies as these regions represent a well-established executive function network (Botvinick et al., 2001; Botvinick et al., 2004; MacDonald et al., 2000; Nieuwenhuis et al., 2003; Simmonds et al., 2008). Although participants were not overtly naming photographs during No-Go trials, we also observed enhanced recruitment of language regions as a function of task difficulty here as well, including bilateral superior and middle temporal gyri, supramarginal gyri, and angular gyri (Indefrey & Levelt, 2000; Poldrack et al., 2001; Poldrack et al., 1999). This may be due to the fact that the linguistic features of the photographs needed to be processed on every trial in order to make the Go/No-Go decisions.
Furthermore, older and younger adults showed some differences in their response to task difficulty in the domain of language production (i.e., Go trials). Behaviorally, older adults showed larger increases in RT as task difficulty increased compared to younger adults. This is consistent with the CRUNCH model (Reuter-Lorenz & Cappell, 2008), and suggests that the effect of task difficulty on behavioral performance may be larger for older adults. With respect to fMRI activation, although there were group differences in overall levels of activation, the two age groups did not show significantly different changes in activation as a function of increased naming or inhibition difficulty within the Bias conditions. These results suggest that older adults had similar neural increases to younger adults when engaged in the Go/No-Go task (e.g., Figure 8). However, group differences emerged when the task changed from basic picture naming to the Go/No-Go task (All Go < Bias conditions), younger adults showed larger increases in naming activation as these general task demands increased compared with older adults. These results suggest that the major age-related differences were driven by the addition of task demands, as opposed to varying the level of difficulty within the Go/No-Go task, which is only partially consistent with the CRUNCH model.
To better understand the nature of age-related differences in behavioral performance and brain activation, we correlated older adults’ fMRI activation and behavioral performance on both Go and No-Go trials. We hypothesized that older adults’ negative fMRI activation-behavior correlations (i.e., faster reaction times or lower error rates associated with more activation) might reflect compensation. On the other hand, positive fMRI activation-behavior correlations (i.e., slower reaction times or higher error rates associated with more activation) or the lack of a significant relationship would suggest reduced neural efficiency (i.e., dedifferentiation or disinhibition). Interestingly, we observed several patterns of activation-behavior correlations. For Go trials in both the All Go condition and the two Bias conditions, negative fMRI activation-behavior correlations were found in right supramarginal and angular gyri, as well as bilateral anterior cingulate, bilateral postcentral gyri, and left precentral gyrus in the bias condition analysis, suggesting a compensatory role for these regions in naming. These regions are either involved in language production or are the right homologues of core language regions (Hickok & Poeppel, 2007; Indefrey & Levelt, 2000, 2004; Price, 2010). Moreover, the beneficial functions of these regions were not unique to older adults. Our previous work in younger adults also showed a compensatory function for bilateral pre- and post- central gyri, as well as right supramarginal gyrus (Zhang et al., 2018), suggesting that engagement of these regions is beneficial to language production across the lifespan. We also observed positive correlations between reaction times and brain activation for older adults in the Bias conditions in bilateral superior frontal gyri, and bilateral precuneus cortex extending to occipital cortex. These results suggest a decline of neural efficiency in these regions since overactivation in older adults was associated with poorer behavioral performance, particularly when additional demands were involved in language production (Li et al., 2001). Interestingly, the precuneus is a prominent region in the default mode network consistent with the idea that enhanced recruitment of Default Model Network regions is detrimental to task performance (Raichle, 2010; Raichle et al., 2001). For language inhibition (i.e., No-Go trials), negative fMRI activation-behavior correlations were found in bilateral lingual gyri extending to into calcarine cortex, suggesting a compensatory role for primary visual cortices during response inhibition for older adults. Positive brain-behavior correlations among older adults were found in left pre- and post-central gyri. Interestingly, this was a region in which we found a negative correlation between RT and activation to overt naming trials. Together these findings suggest that increases in activation in pre- and post-central gyri may facilitate naming, regardless of whether that is the intended response. Finally, we observed reduced correlations between cortical activation and behavior for older adults as general task demands increased for Go trials, and as response inhibition difficulty increased for No-Go trials, suggesting a reduction of the correlations between observed cortical activation and behavior with increased task difficulty, consistent with the CRUNCH model.
Although our results highlight the effects of age and task difficulty on language production, there are a few caveats to the present results. First, there are well-established age-related differences in vasculature and blood flow (De Vis et al., 2015; Jog et al., 2016). In the present study we did not control for potential differences in cerebral blood flow (CBF) or cerebral metabolic rate of oxygen use (CMRO2), which are known to influence the BOLD signal (Buxton, 2013; Buxton, Griffeth, Simon, & Moradi, 2014; Uludağ, Müller-Bierl, & Uğurbil, 2009). Therefore, the group differences in BOLD signal that we observed may be related to CBF/CMRO2 differences between age groups. Second, in the current study, the manipulation of task difficulty for Go trials and No-Go trials was confounded with phoneme monitoring categories (i.e., Go trials for the Go Bias condition always started with consonants, while Go trials for the No-Go Bias condition always started with vowels). There is evidence that these categories have different cortical representations (Bouchard, Mesgarani, Johnson, & Chang, 2013; Obleser, Elbert, Lahiri, & Eulitz, 2003; Obleser, Scott, & Eulitz, 2005) and moreover, that monitoring and processing demands may be different for vowels and consonants (Carreiras & Price, 2008; Sharp, Scott, Cutler, & Wise, 2005). Prior studies have generally found greater processing demands for consonants (i.e., Go trials in the Go Bias runs in the present study), thus any effects of increased difficulty for consonants should work against our main comparisons of interest.
In conclusion, behaviorally, older adults had poorer performance than younger adults in language production and response inhibition. Additionally, older adults showed greater declines in overt naming performance in response to task difficulty than younger adults. Neurally, older adults elicited more extensive patterns of activation than younger adults throughout the brain. Additionally, although we did not see age-related differences in neural activation during the Go/No-Go task, the addition of general task demands (All Go < Bias conditions) to language production affected the two age groups differently, with younger adults eliciting larger increases in activation. More critically, compensatory patterns of activation were found in right supramarginal and angular gyri, as well as bilateral pre- and post- central gyri during overt naming, while activation of bilateral lingual gyri and calcarine cortex was beneficial during response inhibition and attentional monitoring for older adults. Overall, our results are partially consistent with the predictions of CRUNCH model, highlight the interplay between language production and cognitive control, and illustrate how older adults are particularly sensitive to additional task demands.
Supplementary Material
Highlights.
Older adults showed worse performance with increased fMRI activation.
All adults were slower and had more fMRI activation as task difficulty increased.
Behaviorally, older adults were more sensitive to task difficulty.
Compensation was found in bilateral motor and right parietal regions.
Reduced fMRI activation-behavior correlations were found with increased difficulty.
The findings partially support the CRUNCH model.
Acknowledgements
This project was funded by National Institutes of Health (NIH) grant R01 AG034138 (MTD), the Social Sciences Research Institute, and the Department of Psychology at the Pennsylvania State University. We thank Brandi Whyte and Wenjuan Xu for assistance with data analysis. We also thank the staff and scientists at the Social, Life, and Engineering Sciences Imaging Center (SLEIC) and the Center for Language Science (CLS) where the study was conducted, for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Judgments in this task were based on orthographic criteria simply for ease of instruction. We consider this to be a phonological task as participants were required to process phonological aspects of the photographs in order to arrive at correct decisions. Moreover, prior work has demonstrated strong phonological-orthographic mappings in picture naming (Bonin, Peereman, & Fayol, 2001). Additionally, we constrained the items to include only those with regular sound – spelling mappings to ensure a high consistency across orthographic-phonological mappings.
Because participants always switched rules from the 2nd to the 3rd condition, switching costs were a potential concern. Several analyses indicated that these effects were not statistically significant. See supplemental materials—Order Effect: Switching Cost for details.
Data from the norming study showed that the All Go condition stimuli had significantly lower accuracy (80%) compared to the other two lists (91.82% & 91.43%, ps < .001). Because the Bias conditions had more naming requirements (e.g., start with a vowel or a consonant), we selected items with higher naming accuracies for the Bias conditions. Note that this accuracy difference potentially increases the naming difficulty in the All Go condition, however, because we analyzed the All Go condition separately from the Bias conditions, the norming difference between them should not be a major concern.
We used the random function in excel to select subsets of trials from each condition that matched the number of correct responses for each participant (Go trial range: YA: 21–27, OA: 12–25; No-Go trial range: YA: 20–27, OA: 16–26). For example, we selected an equal number of Go trials from the All Go and the Go Bias conditions to match the number of Go trials in the No-Go Bias condition on a subject by subject basis. See supplemental materials—Power Analysis for results.
We also conducted a similar regression analysis on each participant’s reaction time while only including all the two Bias conditions (Go Bias < No-Go Bias), then the regression coefficients were compared between two age groups. Older adults also showed significant greater increase in reaction times from Go Bias to the No-Go Bias condition compared to younger adults (t (35.42) = 2.32, p = .03).
The Stroop effect (also known as Stroop interference) is another measurement of response inhibition. When adding subjects level Stroop effect to the commission error regression model, however, it did not significantly predict the number of commission errors (β = −.59, SE = .36, p = .10). Some literature has challenged the view that Stroop performance represents solely response inhibition or selective inhibition (Shao, Roelofs, Martin, & Meyer, 2015). Rather, it may also depend on the participants’ level of reading automaticity, and can be modulated by task rules, detecting conflict and facilitating the correct response (Leon-Carrion, García-Orza, & Pérez-Santamaría, 2004; MacLeod, Dodd, Sheard, Wilson, & Bibi, 2003; Stuss & Alexander, 2007).
Since participants produced overt speech in the scanner, a potential concern was that this might cause excessive head motion. To address this concern, we compared head movement across the different conditions and between age groups. There were no significant differences between age groups or among different conditions. See supplemental materials—Head Movement for details.
References
- Acheson DJ, Wells JB, & MacDonald MC (2008). New and updated tests of print exposure and reading abilities in college students. Behavior research methods, 40(1), 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TY, & Gold DP (1993). Aging, inhibition, and verbosity. Journal of Gerontology, 48(5), 225–232. [DOI] [PubMed] [Google Scholar]
- Arbuckle TY, Nohara-LeClair M, & Pushkar D (2000). Effect of off-target verbosity on communication efficiency in a referential communication task. Psychology and aging, 15(1), 65–77. [DOI] [PubMed] [Google Scholar]
- Aron AR (2011). From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological psychiatry, 69(12), e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, … Treiman R (2007). The English lexicon project. Behavior research methods, 39(3), 445–459. [DOI] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, & Tily HJ (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of memory and language, 68(3), 255–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823. [Google Scholar]
- Beckmann CF, Jenkinson M, & Smith SM (2003). General multilevel linear modeling for group analysis in FMRI. NeuroImage, 20(2), 1052–1063. [DOI] [PubMed] [Google Scholar]
- Bench C, Frith C, Grasby P, Friston K, Paulesu E, Frackowiak R, & Dolan R (1993). Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia, 31(9), 907–922. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin P, Peereman R, & Fayol M (2001). Do phonological codes constrain the selection of orthographic codes in written picture naming? Journal of memory and language, 45(4), 688–720. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological review, 108(3), 624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences, 8(12), 539–546. [DOI] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, & Chang EF (2013). Functional organization of human sensorimotor cortex for speech articulation. Nature, 495(7441), 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T, Barch D, Keys B, Carter C, Cohen J, Kaye J, … Mumenthaler M (2001). Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General, 130(4), 746–763. [PubMed] [Google Scholar]
- Braver T, Gray J, & Burgess G (2007). Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Variation in working memory, 76–106. [Google Scholar]
- Brodeur MB, Guérard K, & Bouras M (2014). Bank of Standardized Stimuli (BOSS) phase II: 930 new normative photos. PloS one, 9(9), e106953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, & McNeill D (1966). The “tip of the tongue” phenomenon. Journal of verbal learning and verbal behavior, 5(4), 325–337. [Google Scholar]
- Brown S, Laird AR, Pfordresher PQ, Thelen SM, Turkeltaub P, & Liotti M (2009). The somatotopy of speech: Phonation and articulation in the human motor cortex. Brain and cognition, 70(1), 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, & Berman KF (2005). Meta‐ analysis of neuroimaging studies of the Wisconsin Card‐ Sorting task and component processes. Human brain mapping, 25(1), 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, & James LE (2000). Theoretical approaches to language and aging (Perfect TJ & Maylor EA Eds.). New York, NY, US: Oxford University Press. [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, & Wade E (1991). On the tip of the tongue: What causes word finding failures in young and older adults? Journal of memory and language, 30(5), 542–579. [Google Scholar]
- Burke DM, & Shafto MA (2008). Language and aging (Craik F & Salthouse T Eds.). New York: Psychology Press. [Google Scholar]
- Buxton RB (2013). The physics of functional magnetic resonance imaging (fMRI). Reports on Progress in Physics, 76(9), 096601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Griffeth VE, Simon AB, & Moradi F (2014). Variability of the coupling of blood flow and oxygen metabolism responses in the brain: a problem for interpreting BOLD studies but potentially a new window on the underlying neural activity. Frontiers in neuroscience, 8, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and aging, 17(1), 85–100. [DOI] [PubMed] [Google Scholar]
- Carreiras M, & Price CJ (2008). Brain activation for consonants and vowels. Cerebral Cortex, 18(7), 1727–1735. [DOI] [PubMed] [Google Scholar]
- Christensen KJ, Moye J, Armson RR, & Kern TM (1992). Health screening and random recruitment for cognitive aging research. Psychology and aging, 7(2), 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M, & Boulinguez P (2013). Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience & Biobehavioral Reviews, 37(1), 11–23. [DOI] [PubMed] [Google Scholar]
- Dale AM (1999). Optimal experimental design for event-related fMRI. Human brain mapping, 8(2–3), 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, & Cabeza R (2008). Que PASA? The posterior–anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vis J, Hendrikse J, Bhogal A, Adams A, Kappelle L, & Petersen E (2015). Age‐ related changes in brain hemodynamics; A calibrated MRI study. Human brain mapping, 36(10), 3973–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray G, Wilson S, McMahon K, & Muthiah S (2001). The semantic interference effect in the picture‐ word paradigm: An event‐ related fMRI study employing overt responses. Human brain mapping, 14(4), 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Hyman BT (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Hommet C, Domengie F, Boissy J-M, De Marco G, Joanette Y, … Cottier J-P (2012). Influence of age on the dynamics of fMRI activations during a semantic fluency task. Journal of Neuroradiology, 39(3), 158–166. [DOI] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, & Madden DJ (2014). Age-related differences in the neural bases of phonological and semantic processes. Journal of cognitive neuroscience, 26(12), 2798–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, Truong T, & Madden DJ (In Press). Age-related differences in the influence of task-irrelevant information on the neural bases of phonological and semantic processes. Cognitive, Affective, & Behavioral Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Rizio AA, & Zhuang J (2016). The Neural Language Systems That Support Healthy Aging: Integrating Function, Structure, and Behavior. Language and Linguistics Compass, 10(7), 314–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross T, & Stein E (1999). Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences, 96(14), 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Brownsett SL, Leech R, Beckmann CF, Woodhead Z, & Wise RJ (2012). The contribution of the inferior parietal cortex to spoken language production. Brain and language, 121(1), 47–57. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Wise RJ, Mehta A, & Leech R (2014). Overlapping networks engaged during spoken language production and its cognitive control. Journal of Neuroscience, 34(26), 8728–8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P, & Lindenberger U (2003). Age-based structural dynamics between perceptual speed and knowledge in the Berlin Aging Study: direct evidence for ability dedifferentiation in old age. Psychology and aging, 18(4), 696–713. [DOI] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, Van Erp TG, Liu TT, Greve DN, … Keator DB (2012). Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. Journal of Magnetic Resonance Imaging, 36(1), 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Fristen K, & Frackowiak RS (2002). A voxel-based morphometric study of ageing in 465 normal adult human brains. Paper presented at the Biomedical Imaging, 2002. 5th IEEE EMBS International Summer School on. [DOI] [PubMed] [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, & Rypma B (1991). Age and inhibition. Journal of Experimental Psychology: Learning, Memory, and Cognition, 17(1), 163–169. [DOI] [PubMed] [Google Scholar]
- Hasher L, & Zacks RT (1988). Working memory, comprehension, and aging: A review and a new view. Psychology of learning and motivation, 22, 193–225. [Google Scholar]
- Hayasaka S, & Nichols TE (2003). Validating cluster size inference: random field and permutation methods. NeuroImage, 20(4), 2343–2356. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, & Thompson-Schill SL (2006). Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia, 44(12), 2547–2557. [DOI] [PubMed] [Google Scholar]
- Indefrey P, & Levelt WJ (2000). The neural correlates of language production In The new cognitive neurosciences; 2nd ed. (pp. 845–865): MIT press. [Google Scholar]
- Indefrey P, & Levelt WJ (2004). The spatial and temporal signatures of word production components. Cognition, 92(1), 101–144. [DOI] [PubMed] [Google Scholar]
- Jog MA, Yan L, Kilroy E, Krasileva K, Jann K, LeClair H, … Wang DJ (2016). Developmental trajectories of cerebral blood flow and oxidative metabolism at baseline and during working memory tasks. NeuroImage, 134, 587–596. [DOI] [PubMed] [Google Scholar]
- Kemper S, Herman RE, & Lian CH (2003). The costs of doing two things at once for young and older adults: Talking while walking, finger tapping, and ignoring speech of noise. Psychology and aging, 18(2), 181–192. [DOI] [PubMed] [Google Scholar]
- Kemper S, Schmalzried R, Herman R, Leedahl S, & Mohankumar D (2009). The effects of aging and dual task demands on language production. Aging, Neuropsychology, and Cognition, 16(3), 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper S, Schmalzried R, Herman R, & Mohankumar D (2011). The effects of varying task priorities on language production by young and older adults. Experimental Aging Research, 37(2), 198–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper S, Thompson M, & Marquis J (2001). Longitudinal change in language production: Effects of aging and dementia on grammatical complexity and semantic content. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RH (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. [Google Scholar]
- Leon-Carrion J, García-Orza J, & Pérez-Santamaría FJ (2004). Development of the inhibitory component of the executive functions in children and adolescents. International Journal of Neuroscience, 114(10), 1291–1311. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, & Sikström S (2001). Aging cognition: from neuromodulation to representation. Trends in cognitive sciences, 5(11), 479–486. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, & Zacks RT (2007). Inhibitory deficit theory: Recent developments in a “new view”. Inhibition in cognition, 17, 145–162. [Google Scholar]
- Lutkenhoff ES, Rosenberg M, Chiang J, Zhang K, Pickard JD, Owen AM, & Monti MM (2014). Optimized brain extraction for pathological brains (optiBET). PloS one, 9(12), e115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, & Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. [DOI] [PubMed] [Google Scholar]
- MacKay DG, & Burke DM (1990). Chapter five cognition and aging: a theory of new learning and the use of old connections. Advances in psychology, 71, 213–263. [Google Scholar]
- MacKay DG, & James LE (2004). Sequencing, speech production, and selective effects of aging on phonological and morphological speech errors. Psychology and aging, 19(1), 93–107. [DOI] [PubMed] [Google Scholar]
- MacLeod CM (1991). Half a century of research on the Stroop effect: an integrative review. Psychological bulletin, 109(2), 163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, Dodd MD, Sheard ED, Wilson DE, & Bibi U (2003). In opposition to inhibition. Psychology of learning and motivation, 43, 163–215. [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N.-k., & Song AW (2012). Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1822(3), 386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, … Crosson B (2009). Neural signatures of semantic and phonemic fluency in young and old adults. Journal of cognitive neuroscience, 21(10), 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Seeds L, Flaisch T, Harnish S, Cohen ML, McGregor K, … Crosson B (2012). Impact of changed positive and negative task-related brain activity on word-retrieval in aging. Neurobiology of aging, 33(4), 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan O, Coslett H, & Schwartz M (2014). Neural organization of spoken language revealed by lesion-symptom mapping. Nature communications, 6, 6762–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DB (1989). How many memory systems? Evidence from aging. Journal of Experimental Psychology: Learning, Memory, and Cognition, 15(1), 31–49. [DOI] [PubMed] [Google Scholar]
- Monsell S (2003). Task switching. Trends in cognitive sciences, 7(3), 134–140. [DOI] [PubMed] [Google Scholar]
- Moreno-Martínez FJ, & Montoro PR (2012). An ecological alternative to Snodgrass & Vanderwart: 360 high quality colour images with norms for seven psycholinguistic variables. PloS one, 7(5), e37527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CM, Hirsh KW, & Duggan GB (2003). Age of acquisition, ageing, and verb production: Normative and experimental data. The Quarterly Journal of Experimental Psychology: Section A, 56(4), 705–730. [DOI] [PubMed] [Google Scholar]
- Mortensen L, Meyer AS, & Humphreys GW (2008). Speech planning during multiple-object naming: Effects of ageing. Quarterly Journal of Experimental Psychology, 61(8), 1217–1238. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, & Ridderinkhof KR (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience, 3(1), 17–26. [DOI] [PubMed] [Google Scholar]
- Nippold MA, Cramond PM, & Hayward-Mayhew C (2014). Spoken language production in adults: Examining age-related differences in syntactic complexity. Clinical linguistics & phonetics, 28(3), 195–207. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, & Thompson‐Schill SL (2010). Broca’s area and language processing: Evidence for the cognitive control connection. Language and Linguistics Compass, 4(10), 906–924. [Google Scholar]
- Obleser J, Elbert T, Lahiri A, & Eulitz C (2003). Cortical representation of vowels reflects acoustic dissimilarity determined by formant frequencies. Cognitive Brain Research, 15(3), 207–213. [DOI] [PubMed] [Google Scholar]
- Obleser J, Scott SK, & Eulitz C (2005). Now you hear it, now you don’t: transient traces of consonants and their nonspeech analogues in the human brain. Cerebral Cortex, 16(8), 1069–1076. [DOI] [PubMed] [Google Scholar]
- Peramunage D, Blumstein SE, Myers EB, Goldrick M, & Baese-Berk M (2011). Phonological neighborhood effects in spoken word production: An fMRI study. Journal of cognitive neuroscience, 23(3), 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester C-YC, Nelson JK, Welsh KM, Jonides J, & Reuter-Lorenz PA (2004). Selection requirements during verb generation: differential recruitment in older and younger adults. NeuroImage, 23(4), 1382–1390. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, & Ralph MAL (2007). Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences, 104(50), 20137–20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, & Gabrieli JD (2001). Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. Journal of cognitive neuroscience, 13(5), 687–697. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, & Gabrieli JD (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage, 10(1), 15–35. [DOI] [PubMed] [Google Scholar]
- Price CJ (2010). The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences, 1191(1), 62–88. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2010). Two views of brain function. Trends in cognitive sciences, 14(4), 180–190. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL J. P. o. t. N. A. o. S (2001). A default mode of brain function. 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, & Davatzikos C (2003). Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. Journal of Neuroscience, 23(8), 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Cappell KA (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. [Google Scholar]
- Ridderinkhof KR, Van Den Wildenberg WP, Segalowitz SJ, & Carter CS (2004). Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and cognition, 56(2), 129–140. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Schmitt BM, Kutas M, & Münte TF (2002). Electrophysiological estimates of the time course of semantic and phonological encoding during listening and naming. Neuropsychologia, 40(7), 778–787. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Van Der Lugt A, Rotte M, Britti B, Heinze H-J, & Münte TF (2005). Second language interferes with word production in fluent bilinguals: brain potential and functional imaging evidence. Journal of cognitive neuroscience, 17(3), 422–433. [DOI] [PubMed] [Google Scholar]
- Roskies A, Fiez J, Balota D, Raichle M, & Petersen S (2001). Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of cognitive neuroscience, 13(6), 829–843. [DOI] [PubMed] [Google Scholar]
- Schmitt BM, Rodriguez-Fornells A, Kutas M, & Münte TF (2001). Electrophysiological estimates of semantic and syntactic information access during tacit picture naming and listening to words. Neuroscience Research, 41(3), 293–298. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, & Thompson-Schill SL (2009). Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences, 106(1), 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Burke DM, Stamatakis EA, Tam PP, & Tyler LK (2007). On the tip-of-the-tongue: neural correlates of increased word-finding failures in normal aging. Journal of cognitive neuroscience, 19(12), 2060–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Roelofs A, Martin RC, & Meyer AS (2015). Selective inhibition and naming performance in semantic blocking, picture-word interference, and color–word Stroop tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition, 41(6), 1806. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Cutler A, & Wise RJ (2005). Lexical retrieval constrained by sound structure: The role of the left inferior frontal gyrus. Brain and language, 92(3), 309–319. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, & Mostofsky SH (2008). Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia, 46(1), 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Flitney DE (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of experimental psychology, 18(6), 643. [Google Scholar]
- Stuss DT, & Alexander MP (2007). Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society of London B: Biological Sciences, 362(1481), 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, & Burke D (2000). Slips of the tongue: An examination of language production in old age. Paper presented at the Poster presented at the Cognitive Aging Conference, Atlanta. [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Alexander MP, & Stuss D (1998). Clustering and switching on verbal fluency: The effects of focal frontal-and temporal-lobe lesions. Neuropsychologia, 36(6), 499–504. [DOI] [PubMed] [Google Scholar]
- Uludağ K, Müller-Bierl B, & Uğurbil K (2009). An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. NeuroImage, 48(1), 150–165. [DOI] [PubMed] [Google Scholar]
- Van Turennout M, Hagoort P, & Brown CM (1997). Electrophysiological evidence on the time course of semantic and phonological processes in speech production. Journal of Experimental Psychology: Learning, Memory, and Cognition, 23(4), 787. [DOI] [PubMed] [Google Scholar]
- Venables WN, & Smith DM (2006). The R development core team. An Introduction to R. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Visser M, Jefferies E, & Ralph ML (2010). Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. Journal of cognitive neuroscience, 22(6), 1083–1094. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Coalson DL, & Raiford SE (1997). WAIS-III: Wechsler adult intelligence scale: Psychological Corporation; San Antonio, TX. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJG, … Crosson B (2008). Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiology of aging, 29(3), 436–451. [DOI] [PubMed] [Google Scholar]
- Wijeakumar S, Magnotta VA, Buss AT, Ambrose JP, Wifall TA, Hazeltine E, & Spencer JP (2015). Response control networks are selectively modulated by attention to rare events and memory load regardless of the need for inhibition. NeuroImage, 120, 331–344. [DOI] [PubMed] [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, & Holdnack JA (2006). The California Verbal Learning Test–second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of clinical neuropsychology, 21(5), 413–420. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, & Smith SM (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage, 21(4), 1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley K (2001). Statistical analysis of activation images. Functional MRI: An introduction to methods, 14, 251–270. [Google Scholar]
- Zhang H, Eppes A, Beatty-Martinez A, Navarro-Torres C, & Diaz M (2018). Task difficulty modulates brain-behavior correlations in language production and cognitive control: Behavioral and fMRI evidence from a phonological Go – No-Go picture naming paradigm. Cognitive, Affective, & Behavioral Neuroscience, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.