FIG 4.
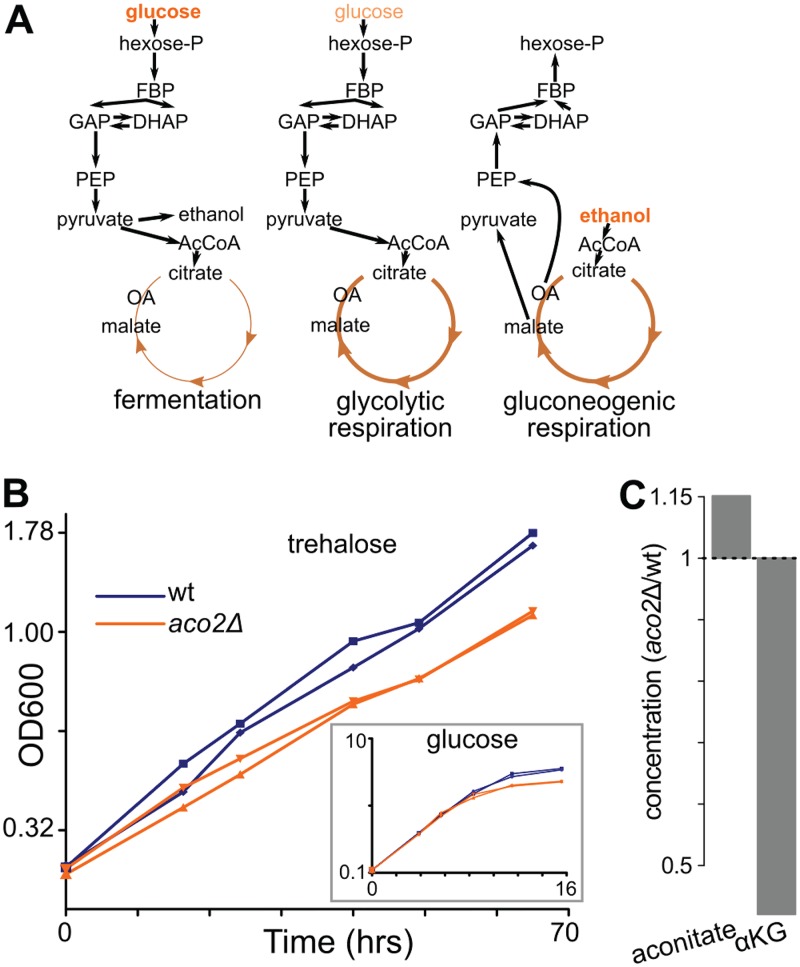
Deletion of the minor aconitase isozyme aco2 results in a selective growth defect on trehalose, indicating impaired glycolytic respiration. (A) Schematic of metabolism across the diauxic shift. In the presence of high levels of glucose (left), S. cerevisiae prefers to ferment glucose to ethanol. As glucose becomes limiting (center), S. cerevisiae continues to use glucose but converts it into acetyl-CoA (AcCoA) and, eventually, CO2, in so doing driving TCA cycle turning and oxidative phosphorylation. We term this state “glycolytic respiration.” Finally, when glucose is exhausted, S. cerevisiae uses ethanol to make acetyl-CoA as well as sugar phosphates through gluconeogenesis. We refer to this state as gluconeogenic respiration. DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde-3-phosphate; FBP, fructose-1,6-bisphosphate; PEP, phosphoenolpyruvate; OA, oxaloacetate. (B) Growth of wild-type (wt) and aco2Δ strains on minimal medium with trehalose, which is digested extracellularly to provide a steady but limiting amount of glucose, revealed a growth defect for the aco2Δ mutant during gluconeogenic respiration. In contrast, under conditions of growth on glucose (inset), the aco2 deletion mutant had no growth defect in the log phase and began to show a growth defect only when glucose became limiting. Data represent biological duplicates. (C) During steady-state growth under conditions of limiting glucose, the level of aconitate was slightly elevated (115% of the wild-type level) and that of α-ketoglutarate decreased (45% of the wild-type level) in the aco2Δ mutant compared to the wild type. Bar plots represent averages of results from four technical replicates (with repeated sampling from one chemostat per strain). αKG, α-ketoglutarate.