Abstract
Rationale:
In patients with pituitary thyroid hormone resistance, the ability of the pituitary gland to detect (and down-regulate) the increase of triiodothyronine is selectively impaired, while the periphery remains sensitive to triiodothyronine levels, producing symptoms of peripheral thyrotoxicity. Subsequently, there is no feedback of pituitary production of thyroid-stimulating hormone (TSH), which is responsible for this hyperthyroidism.
Patient concerns:
We report a case of a 46-year-old Chinese woman diagnosed with a thyroid nodule, with normal thyroid function. She underwent conventional subtotal thyroidectomy, and replacement therapy (levothyroxine) was used for as convention. However, it was later proven that she had pituitary resistance to thyroid hormone, as supra-physiological doses of levothyroxine were required to normalize TSH levels, which resulted in peripheral thyrotoxicity.
Diagnoses:
Based on the patient's symptoms, laboratory tests results, imaging examinations, and genetic analysis (which noted a gene mutation), a diagnosis of pituitary resistance to thyroid hormones was confirmed.
Interventions:
The dose of levothyroxine was adjusted periodically and β-adrenergic blocker was used as symptomatic treatment.
Outcomes:
The outcome in the reported case has been satisfactory despite the persistence of non-suppressed TSH.
Lessons:
An inappropriate level of TSH should always be evaluated. We found a new mutation (H435A) of the thyroid hormone receptor beta gene, which allowed for the establishment of a definitive diagnosis.
Keywords: pituitary thyroid hormone, thyroid hormone, thyroid hormone receptor beta
1. Introduction
Resistance to thyroid hormone (RTH) is a rare, predominantly inherited syndrome, with decreased sensitivity to thyroid hormone (TH), which leads to elevated serum TH concentrations, but inappropriately normal or elevated thyroid stimulating hormone (TSH) concentrations.[1,2] The disease is mostly caused by mutations of thyroid hormone receptor beta (THRB), which is responsible for reduced affinity to the TH receptor. According to genomic studies, the THRB mutation is involved in the molecular pathogenesis of pituitary resistance to thyroid hormone (PRTH).[3] Clinically, RTH was subdivided into 2 different phenotypes; most patients with generalized resistance to thyroid hormone (GRTH) range from asymptomatic to severe symptoms of hypothyroidism. In contrast, patients with PRTH exhibit symptoms of hyperthyroidism, since peripheral tissues are relatively less resistant to TH activity, but are exposed to elevated TH levels.[4]
The occurrence of PRTH is more rare than that of GRTH, and generally, diagnosis of the former is primarily based on the presence of signs and symptoms of hyperthyroidism.[5] Patients may present with tachycardia, thyroid enlargement, weight loss, and other signs or symptoms related to thyrotoxicosis, which is often easily misdiagnosed as hyperthyroidism. In fact, the misdiagnosis of this condition that led to inappropriate therapy or management had previously been reported.[6]
Here, we report the detailed clinical course of a patient with PRTH, who underwent an unnecessary subtotal thyroidectomy. Subsequent genetic analysis revealed a new H435A mutation in exon 10 of the THRB gene.
2. Consent
Written informed consent was obtained from the patient prior to presenting their results in this case report.
3. Case report
A 46-year-old woman was hospitalized in August 2015, with increasing complaints of cervical swelling for the past 2 weeks. She had no history of experiencing hyperthyroidism or symptoms such as irritability, tremor, heat intolerance, fatigue, increased sensitivity to cold, or edema. No features of thyroid-related ocular disease or bone abnormalities were noted either. There was no other significant medical history, including delayed growth and development, or attention deficit hyperactivity disorder (ADHD), or learning disability. Furthermore, neither a history of irradiation nor a family history of thyroid disease was reported.
The first traceable lab test date was August 30, 2015, when she first visited the hospital for “a thick neck,” for which thyroid function tests showed slightly elevated free T3 (FT3), free T4 (FT4), and TSH values. Anti-thyroglobulin antibody (TGAb) and anti-thyroperoxidase (TPOAb) antibody testing were both negative. A mildly enlarged thyroid was found upon palpation, and several nodules were noted. Subtotal thyroidectomy indicated for multinodular goiter was performed. The diagnosis of nodular goiter and thyroid adenoma was confirmed after pathological examination.
On postoperative day 7, levothyroxine (L-T4) replacement therapy at 100 μg daily was started. After 3 weeks of L-T4 therapy, thyroid hormone testing was repeated, resulting in TSH values >100 μUI/mL (reference range, 0.27–4.2 μUI/mL), and FT3 and FT4 values of 3.39 μg/mL (reference range, 4.17.9 μg/mL), and 11.29 μg/mL (reference range, 12–22 μg/mL), respectively.
Thereafter, the dose of L-T4 was increased from 100 to 150 μg daily. Two months after surgery, the hormonal levels remained high (FT3 = 4.2 pg/mL, FT4 = 16.64 pg/mL, and TSH > 100.00 μU/mL), hence the dose of L-T4 was adjusted to 200 μg/d. Three months after surgery, the TSH level dropped to 15.53 μIU/mL, while those of FT4, and FT3 increased to 26.7 μg/mL, and 7.0 μg/mL, respectively.
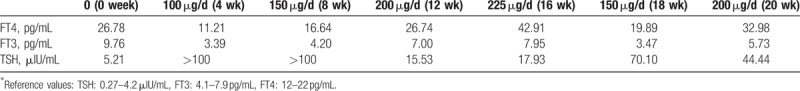
To further control TSH, the L-T4 dose was increased to 225 μg/d. Four months’ postsurgery, the TSH level was 17.93 μIU/mL, which was not significantly change compared with the previous measurement, however, the FT3, and FT4 levels increased drastically (FT3 7.95 pg/mL, FT4 42.91 pg/mL). Meanwhile, the patient began to complain of mild symptoms of hyperthyroidism, including tachycardia and palpitations, as well as anxiety, insomnia, and heat intolerance. To alleviate these symptoms, we decreased the dose of L-T4 to 150 μg/d. In the next 3 weeks, thyroid hormone testing was repeated twice (February 27, 2016, FT3 4.94 pg/mL, FT4 26.50 pg/mL, and TSH 48.09 μU/mL; March 4, 2016, FT3 3.47 pg/mL, FT4 19.89 pg/mL, and TSH 70.1 μU/mL); the hyperthyroidism symptoms disappeared as the TSH level apparently elevated again. When the L-T4 dose was again adjusted to 200 μg/d, the TSH level was not suppressed, despite the high level of FT4 1 month after the last adjustment (FT3 5.73 pg/mL, FT4 32.98 μg/mL, and TSH 44.44 μU/mL). The process of phase change is shown in Table 1.
Table 1.
Thyroid function and the dose change of L-T4 after the surgery.
Due to the high levels of both FT4, and FT3, along with inappropriately TSH levels, as well as the presence of thyrotoxicosis symptoms, the diagnosis of both PRTH, and thyrotropin-secreting pituitary adenomas (TSHomas) were suspected, both of which are considered to be 2 different diseases, with similar consistent thyrotoxic symptoms such as weight loss, tremors, tachycardia and palpitations, anxiety, insomnia, and heat intolerance.
Therefore, the differential diagnosis between PRTH and TSHomas may be difficult. An elevated TSH alpha subunit (α-GSU) concentration, or a high α-GSU/TSH molar ratio favors the diagnosis of TSHoma. Unfortunately, testing for α-GSU was unavailable, and therefore was not performed. The T3 suppression and TRH stimulation tests were not performed either, due to the experimental limitations. However, we noticed that TSH levels were partially suppressed when T3 was above the normal upper limit during the L-T4 dose change; to a certain extent, this favors PRTH. The other hormones were tested, and no significant dysfunction in pituitary hormone secretion was identified.
In addition, pituitary magnetic resonance imaging (MRI) was performed to confirm the current diagnosis. The latter showed a heterogeneously enhanced pituitary, without an obvious lesion, supporting the diagnosis of TSHoma (Fig. 1).
Figure 1.
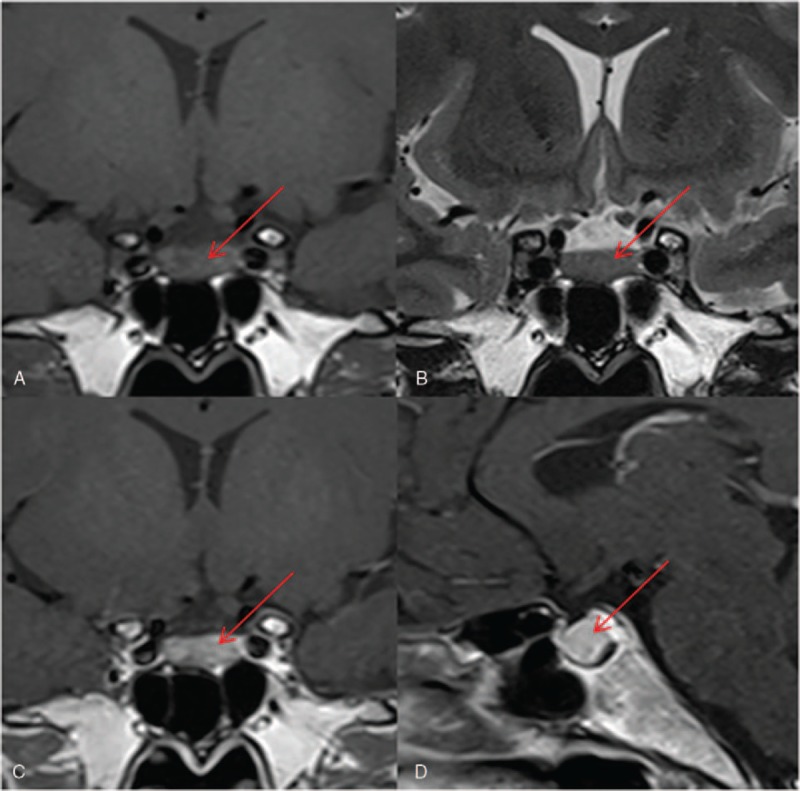
Magnetic resonance imaging (MRI) of the pituitary gland. The pituitary MRI of the patient showed an image that was heterogeneous, and hypointense on coronal T1-weighted imaging (A), and isointense on coronal T2-weighted imaging (B), as observed in the anterior pituitary gland. Sagittal (C) and coronal (D) T1-weighted post-contrast MRI demonstrated a heterogeneously enhancing pituitary.
To further establish definitive diagnosis and explain the possible pathogenesis, analysis of the THRB gene was performed. Genomic DNA was isolated from peripheral blood, and DNA sequence analysis of the THRB gene revealed a heterozygous point mutation (c.1303C>A), leading to a missense change of Histidine 435, to Asparaginate (p.H435A) (Fig. 2). This mutation of THRB (H435A) has never been reported previously. Therefore, the latter supports the hypothesis that our patient was most likely to have PRTH. Genetic analysis of her family members was recommended, but was declined.
Figure 2.
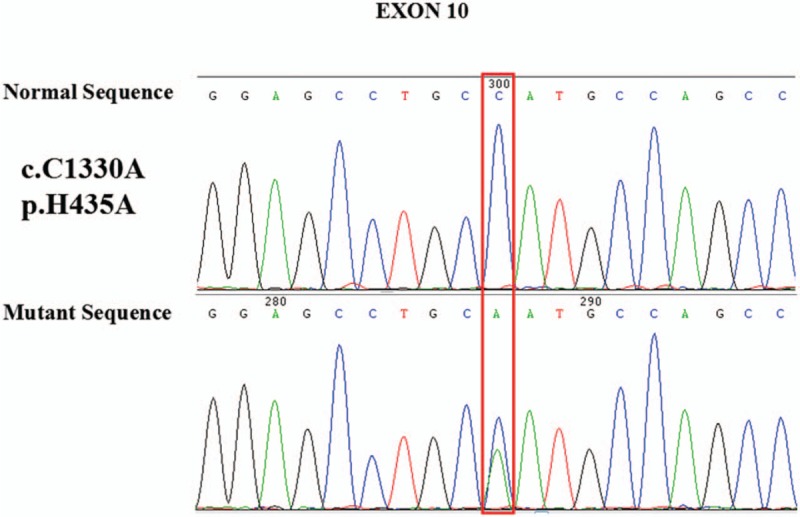
Molecular analysis of thyroid hormone receptor beta in the patient. Partial electropherogram showing the heterozygosity for c.1303C>A missense in exon 10, codon 435.
4. Treatment
Replacement therapy based on periodic dose adjustments of L-T4 was prescribed to lower the TSH levels, and maintain a normal TH level. During the adjustments, when the patient exhibited hyper-adrenergic symptoms such as tachycardia and palpitations, sweating, tremors, weight loss or irritability, β-adrenergic blockers (e.g., propranolol) were used to control these symptoms, and the L-T4 dose was reduced. Yearly thyroid ultrasonography and MRI of the pituitary gland are required. Therapeutically, the patient had maintained clinically stable TSH levels (albeit slightly elevated), and mildly elevated FT4 and FT3, with no complaint of hyperthyroidism symptoms, until the submission date. This case report was approved by the ethics committee of Shandong Provincial Qianfoshan Hospital, Jinan, China, and written informed consent was obtained (2017S109).
5. Discussion
RTH is a rare disorder characterized by reduced target tissue responsiveness to thyroid hormones, usually caused by mutations in THRB. While this was first reported by Refetoff in 1967, now several 100 mutations have been identified.[2,7] The THRB is expressed in the hypothalamus and pituitary gland, and plays an important role in the feedback regulation of thyroid hormone levels.[8] RTH caused by the mutation in THRB is characterized by elevated serum TH levels, associated with normal or elevated TSH, which can be classified into 2 entities according to the clinical presentation: GRTH and PRTH. While patients with GRTH typically a demonstrate euthyroid or hypothyroid pattern, those with PRTH are usually clinical defined as being patients with hyperthyroidism.[9,10]
In our patient, elevated TH levels with non-suppressive levels of TSH, symptoms related to thyrotoxicosis, exclusion of a TSHomas by MRI, and the presence of a rare mutation, H435A, in the THRB support the diagnosis from PRTH. In addition, pituitary resistance was confirmed when the patient needed supra-physiological doses of L-T4 to produce the expected suppressive effect on TSH secretion, but low doses on metabolic responses in peripheral tissues.
Patients with PRTH are the most difficult to manage. When hyperthyroid features were exhibited at the tissue level (as observed in our patient), the elevated TH levels generally require treatment to be controlled within the normal range. Triiodothyroacetic acid (TRIAC) is a physiological metabolite of T3 that has been found to be useful in the treatment of RTH, reducing the TSH and endogenous TH serum levels, and alleviating symptoms.[11–13] However, TRIAC is not available for our patient and her symptoms of peripheral thyrotoxicosis was relieved after treatment with propranolol. No specific treatment has been found to correct the defect completely so far.
Regarding patients with PRTH who have undergone thyroidectomy, who need L-T4 replacement therapy (such as our patient), it is important to realize the sole purpose of normalizing the TSH and TH levels. Sometimes the clinical status and mimicking as much as possible of the preoperative hormonal pattern for T4 and T3 are more significant than optimal suppression of TSH which is often difficult to obtain. Other drugs that are of interest are those that can suppress TH concentrations by suppressing TSH. However, somatostatin analogues have been reported to have limited effects due to their partial effects and inability to maintain TSH suppression.[14]
In conclusion, we have reported the case of a Chinese adult woman with PRTH syndrome, in whom we found a novel mutation (H435A) of the THRB gene. The diagnosis of this syndrome was based on a combination of clinical manifestations, laboratory tests, and genetic evaluations. Our findings provide insight into the diagnosis of PRTH syndrome, and bring more attention to this disease in clinical practice in patients with an inappropriate TSH level.
Author contributions
Data curation: Changzhen Yu, Jinming Yao, Lin Liao.
Formal analysis: Changzhen Yu.
Investigation: Changzhen Yu, Hongxia Shang, Yujiao Cui, Likang Wang, Lin Liao.
Methodology: Changzhen Yu, Lin Liao.
Writing – original draft: Changzhen Yu.
Writing – review and editing: Junyu Zhao.
Conceptualization: Huanjun Wang, Jianjun Dong, Lin Liao.
Software: Rui Zhang.
Funding acquisition: Jianjun Dong, Lin Liao.
Resources: Jianjun Dong, Lin Liao.
Footnotes
Abbreviations: FT3 = free T3, FT4 = free T4, GRTH = generalized resistance to thyroid hormone, L-T4 = levothyroxine, MRI = magnetic resonance imaging, PRTH = pituitary resistance to thyroid hormone, RTH = resistance to thyroid hormone, TGAb = anti-thyroglobulin antibody, TH = thyroid hormone, THRB = thyroid hormone receptor beta, TPOAb = anti-thyroperoxidase, TRIAC = triiodothyroacetic acid, TSH = thyroid stimulating hormone, TSHomas = thyrotropin-secreting pituitary adenomas.
CY and JZ are equal contributors.
The funding body of this National Natural Science Foundation of China Grants (81070637, 81570742, 81770822) in the design of the study and collection, and in writing the manuscript played an important role.
No competing financial interests exist.
References
- [1].Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev 1993;14:348–99. [DOI] [PubMed] [Google Scholar]
- [2].Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab 2007;21:277–305. [DOI] [PubMed] [Google Scholar]
- [3].Lee S, Young BM, Wan W, et al. A mechanism for pituitary-resistance to thyroid hormone (PRTH) syndrome: a loss in cooperative coactivator contacts by thyroid hormone receptor (TR)beta2. Mol Endocrinol 2011;25:1111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mitchell CS, Savage DB, Dufour S, et al. Resistance to thyroid hormone is associated with raised energy expenditure, muscle mitochondrial uncoupling, and hyperphagia. J Clin Invest 2010;120:1345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beck-Peccoz P, Chatterjee VK. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid 1994;4:225–32. [DOI] [PubMed] [Google Scholar]
- [6].Olateju TO, Vanderpump MP. Thyroid hormone resistance. Ann Clin Biochem 2006;43(Pt 6):431–40. [DOI] [PubMed] [Google Scholar]
- [7].Weiss RE, Refetoff S. Resistance to thyroid hormone. Rev Endocr Metab Disord 2000;1:97–108. [DOI] [PubMed] [Google Scholar]
- [8].Flamant F, Baxter JD, Forrest D, et al. International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev 2006;58:705–11. [DOI] [PubMed] [Google Scholar]
- [9].Kong AP, Lam CW, Chan AO, et al. Resistance to thyroid hormone in a Chinese family with R429Q mutation in the thyroid hormone receptor beta gene. Hong Kong Med J 2005;11:125–9. [PubMed] [Google Scholar]
- [10].Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta 2013;1830:3987–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lafranchi SH, Snyder DB, Sesser DE, et al. Follow-up of newborns with elevated screening T4 concentrations. J Pediatr 2003;143:296–301. [DOI] [PubMed] [Google Scholar]
- [12].Sugita M, Harada H, Yamamoto T. Perioperative management of a patient with thyroid hormone resistance who underwent total thyroidectomy for thyroid cancer. J Anesth 2012;26:595–7. [DOI] [PubMed] [Google Scholar]
- [13].Kunitake JM, Hartman N, Henson LC, et al. 3,5,3′-triiodothyroacetic acid therapy for thyroid hormone resistance. J Clin Endocrinol Metab 1989;69:461–6. [DOI] [PubMed] [Google Scholar]
- [14].Dong Q, Gong CX, Gu Y, et al. A new mutation in the thyroid hormone receptor gene of a Chinese family with resistance to thyroid hormone. Chin Med J (Engl) 2011;124:1835–9. [PubMed] [Google Scholar]


