Abstract
The aim of this study is to estimate the risk factors of both respiratory complication (RC) and mortality after acute traumatic cervical spinal cord injury (TCSCI). Between July 2005 and July 2015, in 181 patients (142 males and 39 females; mean age 41.0 years) with acute TCSCI, we compared the difference and odds ratio in RC group (n = 73) with that of non-RC group (n = 108), and also death group (n = 15) and survival group (n = 166). We collected injury-related information after half a year of injury, which is as follows: the causes of injury, time of surgery, ICU (intensive care unit) days, ventilator days, ASIA (American Spinal Injury Association) classification, neurological injury, CIPS (Clinical Pulmonary Infection Score), and BMI (body mass index). Besides these, we gathered the general information such as age, gender, smoking history, and use of steroids. The study compared perioperative parameters; surgery-related and instrumentation- and graft-related complication rates; clinical parameters; patient satisfaction; and radiologic parameters. Variations like gender (odds ratio [OR] = 1.269, 95% confidence interval [CI] [0.609–2.646]), smoking history (OR = 2.902, 95% CI [1.564–5.385]), AIS grade (grade A) (OR = 6.439, 95% CI [3.334–12.434]), neurological level (C1-C4) (OR = 2.714, 95% CI [1.458–5.066]), and steroid use (OR = 2.983, 95% CI [1.276–6.969]) have a facilitated effect on RC. When we estimated surgery-related affection, only the time of surgery and anterior approach compared with posterior has significant difference in RC (P < .05). Between death and survival group, the aspect of age, non-surgical, CPIS, AIS grade, and BMI have statistically significant difference. Survival analysis reveals significant difference in aforementioned groups. In patients suffering from acute TCSCI, those who are old, have long smoking history, complete spinal cord injury, C1-C4, high CPIS, and fat have high incidence of RC and mortality.
Keywords: follow-up, mortality, outcome, respiratory complication, risk factor, traumatic cervical spinal cord injury
1. Introduction
The severe acute traumatic cervical spinal cord injury, (TCSCI) in addition to causing the dysfunction of sensation and movement below the level of injury, leads to paralysis of the diaphragm (innervated by C3-C5 segments) and decreases the motion of respiratory muscles,[1] which have a considerably greater impact than cardiac activity,[2] and need intervention of mechanical ventilation. These respiratory complications (RCs) always cause significant mortality.[3]
Patients with complete spinal cord injury, neurological level above C4, and operative treatment were predisposed to risk factors related to the need for mechanical ventilation in isolated acute TCSCI patients.[4,5] Apart from the aforementioned, most of the researchers hold that many factors influence the morbidity and mortality such as age, cause of injury, use of steroid, surgery intervention, and ICU (intensive care unit) days.[6,7] Ventilation (intubation, tracheotomy, and tracheostomy) is always conducted instantly[8] after RC (which includes pneumonia, atelectasis, and hemothorax) occurs to improve the situation and avoid further aggravation; the intermediate respiratory care unit (IRCI) could solve this problem.[9] According to the aforementioned, early detection and timely treatment, particularly, are more important; the efficiency of predictable risk factors must be evaluated in real-time and side-by-side.
Surgery of acute TCSCI is performed according to the overall condition of patients; most of the orthopedists or neurosurgeons advocate that the early decompression could obtain lower rate of RC[10] and improve outcomes.[11] But the controversy of the time of surgery still exists. Beyond that, the surgery approach of fixation and fusion cannot be ignored, especially the operative route of anterior, posterior, or both. Few researches have studied the relationship between approach route and morbidity and mortality of RC.[12] Thus, the route which would receive less RC and mortality needs to be studied.
The purpose of the study is to estimate the deficiency of as many risk factors as possible that we could gather with limited information on RC and mortality after acute TCSCI; which is beneficial for the treatment and outcome. Furthermore, we also tried to determine the best operation time and approach route that decreases RC and mortality. Thereby provide the evidence of treating acute TCSCI concomitant with RC.
2. Methods and materials
2.1. Patient population
All study methods were approved by the Ethics Committee of the First Affiliated Hospital of PLA General Hospital and the First Affiliated Hospital of Dalian Medical University. All the subjects that were enrolled into the study gave formal written consent to participate. A retrospective study was done to analyze 206 cases of acute TCSCI from 2005 to 2015 in the First Affiliated Hospital of PLA General Hospital and the First Affiliated Hospital of Dalian Medical University. And after we excluded those without neurological impairment (AIS grade E), 181 patients met with the condition (Fig. 1).
Figure 1.
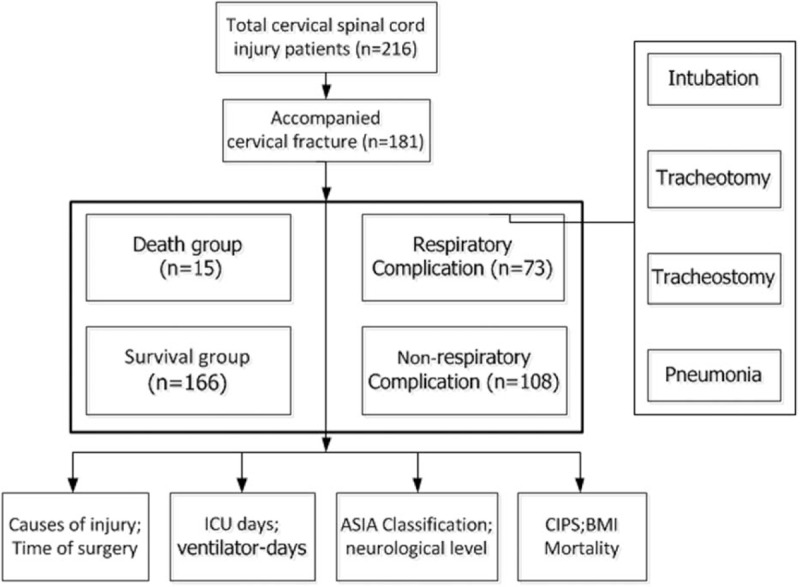
Technology roadmap.
The collected injury-related information after half a year of injury is as follows: the causes of injury, time of surgery, ICU days, ventilator days, ASIA (American Spinal Injury Association) classification, neurological injury, CIPS (Clinical Pulmonary Infection Score), and BMI (body mass index). Besides that, we gathered general information such as age, gender, smoking history, and use of steroids. We then analyzed data as follows. First, we divided the total number of patients into CR group and non-CR group, according to the presence or absence of respiratory complication. CR was defined by single or more symptoms, that is, intubation, tracheotomy, tracheostomy, and pneumonia. If patient needed intervention of mechanical ventilation to breathe, and atelectasis and hemothorax attributed to pneumonia, then we compared pneumonia with the rest of three. Second, we grouped patients into death and survival groups, and then, we did contrastive analysis to find the difference between them.
It is needed to clarify the following concepts: MVA (motor vehicle accident), fall (injury from falling), and others are the causes of TCSCI; the grade of neurological assessment of ASIA classification referred to in Table 1, which constitutes degree of complete (A) and incomplete (B/C/D); the neurological impairment level is defined as upper level (>C4) and lower level (<C4); neurological level is the last normal level of sensory and motor function; 1000 mg of intravenous methylprednisolone less than 8 hours as high-dose steroid; CPIS is a clinical score of 0 to 12 based on the following 6 parameters: body temperature, blood leukocyte level, volume and character of tracheal secretions, arterial oxygenation, pulmonary radiograph findings, and results of culture of tracheal aspirate specimens.
Table 1.
ASIA classification.

2.2. Statistical analysis
Descriptive statistics were performed for all variables. Data was normally distributed; the means and standard deviations were reported and compared using t tests. Continuous variables were reported as medians with interquartile ranges (IQRs) and compared using the nonparametric Mann-Whitney U test. Categorical variables were compared using chi-square test or Fisher exact test. P < .05 was considered to be statistically significant, 95% confidence interval (CI) and odds ratio (OR) were calculated when appropriate. Data were analyzed by the IBM Statistical Program for Social Sciences (version 19.0 software) and GraphPad prism software (version 5.0 software).
3. Results
3.1. General information
During a short time of half-year follow-up after the injury, and exclusion of those without neurological impairment (AIS grade E, n = 20) and loss of follow-up (n = 5), a total of 181 cases were maintained. Among these patients, average age was 41 ± 15 years; male/female ratio was 142/39; 91 cases (50.28%) had smoking history; 95 (52.49%), 51 (28.18%), and 35 (19.33%) were cases of injury because of MVA, fall, and others, respectively; 75 (41.44%) patients had complete degree of injury (AIS grade A); based on the neurological level, there were 98 (54.14%) patients in upper neurological level and 83 (45.86%) in lower; 37 (20.44%) had comorbidities; average CPIS was 4.80 ± 3.20; average BMI was [p25 = 19.57, p75 = 23.97] p50 = 21.83; ICU duration was of 5.33 ± 15.64 days; 144(79.56%) used high-dose steroid; and 15 (8.29%) suffered mortality.
3.2. Difference between RC and non-RC groups
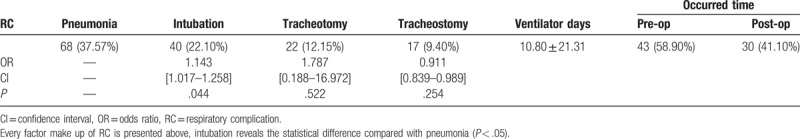
The data of 73 (40.33%) and 108 (59.67%) patients were analyzed for RC group and non-RC group, respectively. There were statistically significant differences in smoking history, AIS grade, injury level, CPIS, BMI, ICU days, and mortality between the groups (P < .001). The use of high-dose steroid also has statistical difference (P < .05). Among all variables compared between RC group and non-RC group, the odds ratio more than 1.0 was gender (OR = 1.269, 95% CI: 0.609–2.646), smoking history (OR = 2.902, 95% CI [1.564–5.385]), AIS grade (OR = 6.439, 95% CI [3.334–12.434]), injury level (OR = 2.714, 95% CI [1.458–5.066]), and steroid use (OR = 2.983, 95% CI [1.276–6.969]), which means the aforementioned factors have a facilitated effect on the RC in patients with acute TCSCI (Table 2). According to pneumonia, intubation, tracheotomy, and tracheostomy, the prevalence of RC in 181 patients of our study was 68 (37.57%), 40 (22.10%), 22 (12.15%), and 17 (9.40%), respectively (Table 3).
Table 2.
Comparison between RC and non-RC.
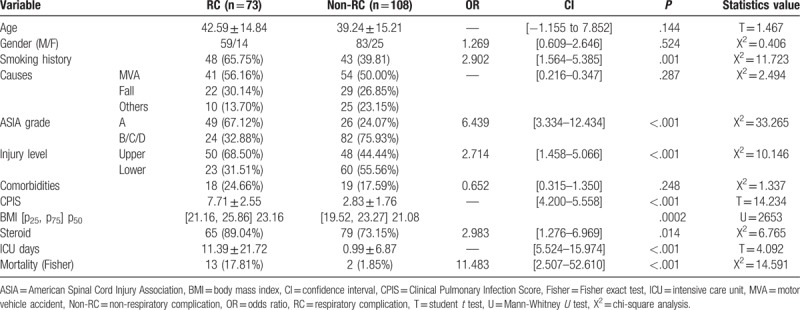
Table 3.
Respiratory complications.
3.3. Surgery-related factors between RC and non-RC groups
In all patients, there were 33 (18.23%) fracture patients accompanied with dislocation, and 35 (19.34%) patient without operation, surgery compared with non-surgery has statistically significant difference (P = .001 < .01). The average time of surgery after injury was 9.12 ± 10.83 days. Only the time of surgery and the anterior approach operation compared with posterior have significant difference between RC and non-RC groups (mean: 6.57 ± 8.19 days) (Table 4). Thus, we found that early surgery intervention could reduce the occurrence of respiratory circumstance (Fig. 2).
Table 4.
Surgery-related factors between the group of RC and non-RC.
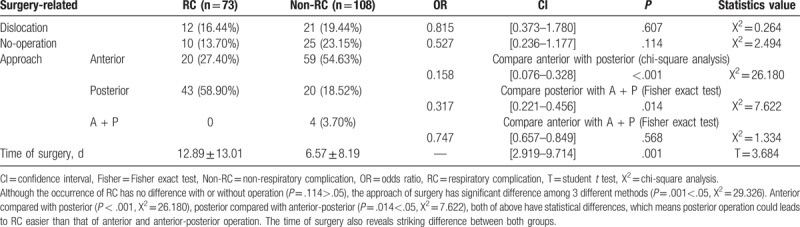
Figure 2.
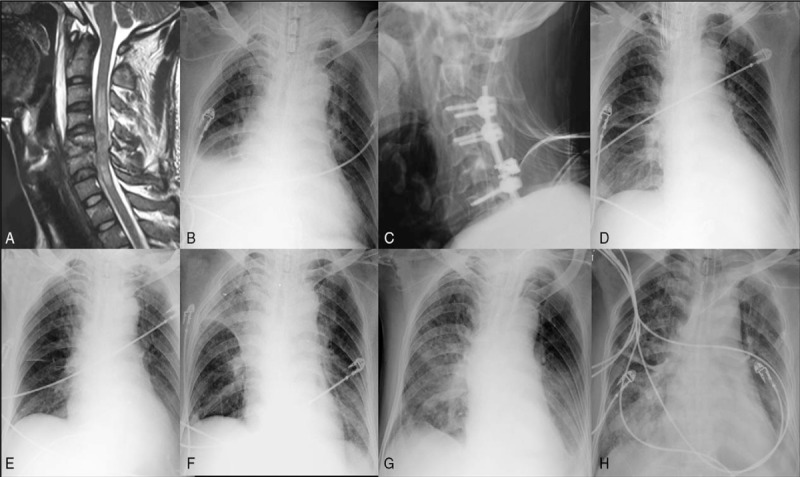
An acute TCSCI patient (ASIA grade A level, neurological level C5-C6), had atelectasis of left lung before surgery (A); 1 day after surgery of anterior cervical discectomy and fusion, the left lung recruitment maneuver was done (B); the condition of left lung became worse after patient was transferred from ICU to the general ward of our department, because of being bed-ridden for a few days (C, D, E, F); after disciplinary respiratory muscle training and the recovery of pulmonary function, patient got well (E, F). TCSCI = traumatic cervical spinal cord injury.
3.4. Difference between death and survival groups
Fifteen patients’ death (8.29%) occurred within half years of injury; among them, 2 (1.10%) patients were without RC, and male/female ratio was 13/2. The percentage of MVA, fall, and blunt injury based on the death of patients was 10 (66.67%), 4 (26.67%), and 1 (6.66%), respectively. There was only 1 person without use of steroid in the acute phase. More than half of the patients in death group had no surgery (60%). Ten patients (66.67%) had upper cervical fracture; death time after injury was 16.73 ± 17.21 days, except in 5 cases (33.33%) in which it was 14.4 ± 11.96 days as the treatment was abandoned because of the inability to bear the cost. The first 3 cause of death were respiratory failure (60%, 9/15), electrolyte disturbances (40%, 6/15), and organ dysfunction (26.67%, 4/15). Respiratory failure included hemothorax, pulmonary embolism, and atelectasis. In sequence, common electrolyte disturbances were hyponatremia, hypochloremia, and hyperkalemia. The detailed information about causes of death is presented in Table 5. Furthermore, we divided total patients into death and survival group, and then, compared each of their variables like aforementioned. It is obvious that there was statistically significant difference between the 2 groups in the aspect of age, non-surgical, CPIS and AIS grade (P < .001), and BMI (P = .041 < .05). In all, it pointed the factors that were responsible for mortality (Table 6).
Table 5.
Demographic data of death patients.
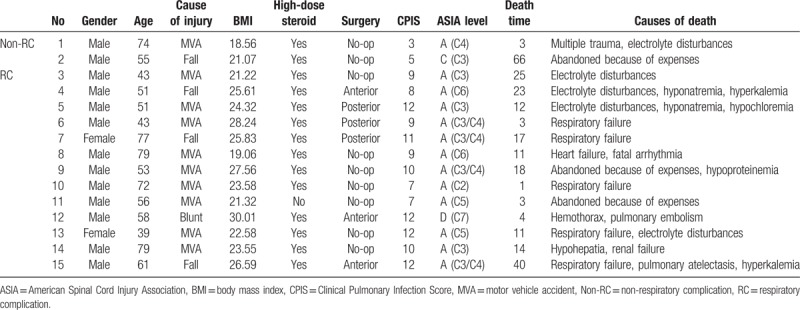
Table 6.
Comparison between death group and survival group.
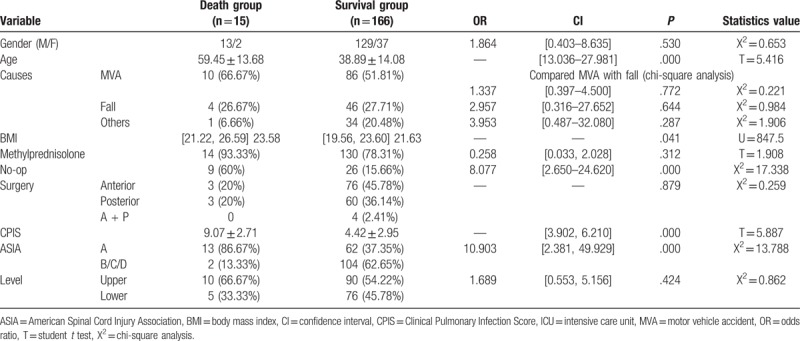
When we made survival analysis according to RC, with statistically significant difference (P = .0026 < .01, log-rank test), it revealed that RC could lead to death compared with non-RC (Fig. 3A). We applied the same statistics method, and found that the death rate of non-surgery patients was significantly lower than that of surgery group (P < .001), whereas no difference existed among the groups of different surgical approach (Fig. 3B).
Figure 3.
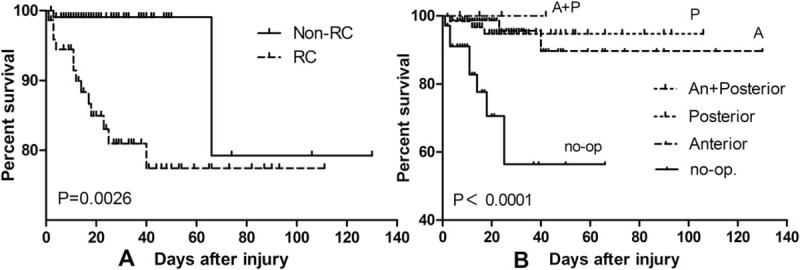
The survival analysis (log-rank test) between RC and non-RC (A), and also the group of surgery and non-surgery (B).
4. Discussion
In this study, we have analyzed retrospectively the difference between acute TCSCI patients who had RC and those without, in short-term period, and surgery and mortality related to it was also analyzed.
A total of 181 patients grouped according to RC, age, gender, cause of injury, and comorbidities had no differences statistically. However, smoking history, AIS grade, neurological level, CPIS, BMI, steroid use, and ICU days have significant difference, which is similar to findings in previous studies.[13] We found that the most-related factors were AIS grade, steroid use, smoking history, and neurological level. A multivariate logistic regression study considered that complete injury was an independent predictor for RC.[14,15] Yugué identified age >69 years was a significant risk factor, which contradicted with our findings. Regarding the affections of neurological level on RC, Berney holds that it is a significant risk factor.[16] We persisted that the higher the level of fracture occurred, the easier the RC happened, especially in injuries above C4. Jackson and Tollefsen believe that 75% to 84% tetraplegia above C4 will experience respiratory problems that requires invasive mechanical ventilation.[17,18] However, Thomas's research found that patients with traumatic lower CSCI would have a high rate of pneumonia during the first 4 weeks after injury.[19] In clinical practice, the CPIS score is always utilized to assess pulmonary infection.[20] In our study, we utilized CPIS to evaluated pneumonia objectively and synthetically. Controversy still exists in China regarding the use of steroid; many clinicians found that Suberviola B did not increase the chances of mortality, but was associated with increased respiratory tract infection.[21]
Our study confirmed that the respiratory management of acute TCSCI is essential for every subsequent treatment, especially for complete (AIS grade A) and upper cervical fracture patients (C4 or higher). Victor J consider that early intubation is mandatory for CSCI patients, and incomplete patients should receive immediate airway intervention, 50% of them require tracheostomy,[22] and Kornblith LZ advocated a short-term mechanical ventilation too.[23] In our study, 68 (37.57%), 40 (22.10%), 22 (12.15%), and 17 (9.4%) patients had pneumonia, intubation, tracheotomy, and tracheotomy, respectively, in a short period of time after injury. Researcher believe in case of both complete and upper level injury, the first 4 weeks after TCSCI were identified for the development of pneumonia and/or duration of ventilation besides pre-existing general conditions.[19,24] Berney S concluded that intubation, mechanical ventilation, and tracheostomy are the mainstay of respiratory management for complete injuries above the level of C5.[16] Therefore, it should be performed timely before the pejoration of respiratory situation. Branco BC regarded that the complete CSCI, facial fracture, and thoracic trauma were independently associated with the need for tracheostomy.[15] Magnetic resonance imaging (MRI), especially delayed MRI, can accurately localize CSCI and give useful information about the state of the spinal cord that needs mechanical ventilation or other intervention.[25,26] Diffusion tensor imaging (DTI) are available, which may be particularly useful in clinical assessment as a marker to assess the level of injury, and the indices named FA (fractional anisotropy) give significant correlation with clinical grade, at the level of injury, if FA value is significantly decreased while MD (mean diffusivity) value is significantly increased.[27] In contrast, Battistuzzo holds that the radiological investigation have no influence on the timing of surgery.[28] However, healthcare/ventilator-associated pneumonia should be taken into consideration. It is reported that 50% patients developed ventilator-associated pneumonia, and 90% occurred before tracheostomy, whereas the author concluded that early tracheostomy can be performed safely after cervical spine fixation surgery.[29]
The early surgical management in this study proved within 1 week, and the approach prioritized choosing anterior cervical spine fixation. Many authors found that early decompression either <24 or <72 hours resulted in statistically better outcomes compared with delayed decompression.[30–32] For incomplete patients, early decompression might receive neurological improvement.[33] However, Liu Y concluded a contradictory outcome that early surgical intervention had higher incidence of mortality and neurological deterioration compared with late surgical intervention and advocated that it might be safe after the first 72 hours.[34] In past decades, a great improvement in instruments and approach have made possible effective fixation and fusion of nearly all TCSCI. The choice of procedure might be determined by the comorbidities, area of compression, type of deformity, and surgeon preference.[35] When treating multilevel cervical spinal cord injury that is complicated with unstable fracture, the open-door expansive laminoplasty combined with posterior transpedicular screw fixation is beneficial.[36] In our experience, once the patient in a stable condition for surgery, early decompression (72 hours) and anterior fixation and fusion could decrease RC, because the anterior approach requires a simple position, short time, and less bleeding in surgery, and less fixed segment, when compared with posterior approach, which requires prone position (harmful for pulmonary function), long time, and more bleeding in surgery.
In our study, high rate of mortality also exists; the mean age of these patients was 59.45 ± 13.68 years, which is remarkably more than survival group. Previous studies have confirmed cervical SCI in patients severely compromises respiratory function,[37] which has been reported to account for 80% of deaths in patients with cervical SCI,[16,38] and 38% in-hospital mortality rate.[39] The most common cause of death in our study was respiratory failure that is identified with previous studies; tracheostomy is associated with major morbidity.[23] Pre-existing general conditions and age did not associate with in-hospital mortality.[3] It should not be neglected that electrolyte disturbances was the second highest cause of death in our study, which sequentially involves hyponatremia, hypochloremia, and hyperkalemia. The cost of treatment was also the reason that led patients and family to give up.[40] Some risk factors affecting mortality, in our study, were age, BMI, no-operation, CPIS, and the grade of AIS. The strongest risk factors for mortality were severity of SCI and injury level in elderly patients,[3] though in our result, the level of injury was not a risk factor. If patients are overweight, the mortality is also higher.
Because of paralysis, impairment of the respiratory muscles, reduced pulmonary vital capacity, and impairment in the clearance of tracheobronchial secretions, it was necessary to prevent RC, after intubation in acute phase of TCSCI; it was difficult to remove on account of reduced hypercapnia drive response.[41] Thus respiratory muscle training is effective for increasing respiratory muscle strength and perhaps also lung volumes for people with cervical SCI.[42]
5. Conclusion
In patients suffering from acute TCSCI, vigilance is required for those who are old, have smoking history, complete SCI, upper injury level, high CPIS, and obesity, when these patient present with pneumonia, atelectasis, and hemothorax. Timely intervention of mechanical ventilation such as intubation, tracheotomy, and tracheostomy is indispensable. Early surgical treatment is inevitable within 1 week of injury; priority should be given to anterior fixation and fusion surgery if without contraindication. ICU or IRCI and an experienced physician are necessary to prevent exacerbation or death because of respiratory failure and electrolyte disturbance.
5.1. Limitation
Our study has several limitations. First, it was a retrospective study. Second, 181 patients were relatively small sample size. Third, in respiratory group, we ignored pulmonary function test (PFT) despite considering CPIS. Other researchers performed mechanical ventilation which was based on the results of PFT, for example, forced vital capacity (FVC) less than 830 mL (11.9 mL/kg) and %VC <16.3% has been reported to be the most important factor for tracheostomy.[14,43] Fourth, we did not describe the detailed methods about the surgery, which might affect the outcomes. Finally, the detail about intensive respiratory care was acquired from different hospital and clinicians, which needs a multicenter clinical trial.
Acknowledgments
We kindly appreciate Zhonghai Li PhD for his valuable help to the study designing of the paper, and thank Jiaguang Tang for language editing.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CIPS = Clinical Pulmonary Infection Score, ICU = intensive care unit, IRCI = intermediate respiratory care unit, MVA = motor vehicle accident, OR = odds ratio, RC = respiratory complication, TCSCI = traumatic cervical spinal cord injury.
X-xY and Z-qH contributed equally to the manuscript and should be considered co-first authors.
All authors state that there are no actual or potential of conflicts of interest in relation to this article
References
- [1].Krassioukov A. Autonomic function following cervical spinal cord injury. Respir Physiol Neurobiol 2009;169:157–64. [DOI] [PubMed] [Google Scholar]
- [2].Winklhofer S, Schoth F, Stolzmann P, et al. Spinal cord motion: influence of respiration and cardiac cycle. RoFo 2014;186:1016–21. [DOI] [PubMed] [Google Scholar]
- [3].Daneshvar P, Roffey DM, Brikeet YA, et al. Spinal cord injuries related to cervical spine fractures in elderly patients: factors affecting mortality. Spine J 2013;13:862–6. [DOI] [PubMed] [Google Scholar]
- [4].Tanaka J, Yugue I, Shiba K, et al. A study of risk factors for tracheostomy in patients with a cervical spinal cord injury. Spine 2015;41:764–71. [DOI] [PubMed] [Google Scholar]
- [5].Lertudomphonwanit T, Wattanaapisit T, Chavasiri C, et al. Risk factors relating to the need for mechanical ventilation in isolated cervical spinal cord injury patients. J Med Assoc Thai [Chotmaihet Thangphaet] 2014;97suppl 9:S10–5. [PubMed] [Google Scholar]
- [6].Yu WK, Ko HK, Ho LI, et al. Synergistic impact of acute kidney injury and high level of cervical spinal cord injury on the weaning outcome of patients with acute traumatic cervical spinal cord injury. Injury 2015;46:1317–23. [DOI] [PubMed] [Google Scholar]
- [7].Song J, Shao J, Qi HH, et al. Risk factors for respiratory failure with tetraplegia after acute traumatic cervical spinal cord injury. Eur Rev Med Pharmacol Sci 2015;19:9–14. [PubMed] [Google Scholar]
- [8].Grant RA, Quon JL, Abbed KM. Management of acute traumatic spinal cord injury. Curr Treat Options Neurol 2015;17:334. [DOI] [PubMed] [Google Scholar]
- [9].Romero-Ganuza J, Garcia-Forcada A, Vargas E, et al. An intermediate respiratory care unit for spinal cord-injured patients. A retrospective study. Spinal Cord 2015;53:552–6. [DOI] [PubMed] [Google Scholar]
- [10].McKinley W, Meade MA, Kirshblum S, et al. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehab 2004;85:1818–25. [DOI] [PubMed] [Google Scholar]
- [11].Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PloS One 2012;7:e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ogungbo B. Anterior decompression, fusion and plating in cervical spine injury: early experience in Abuja, Nigeria. Surg Neurol Int 2011;2:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen Y, Shao J, Zhu W, et al. Identification of risk factors for respiratory complications in upper cervical spinal injured patients with neurological impairment. Acta Orthop Traumatol Turc 2013;47:111–7. [DOI] [PubMed] [Google Scholar]
- [14].Yugue I, Okada S, Ueta T, et al. Analysis of the risk factors for tracheostomy in traumatic cervical spinal cord injury. Spine 2012;37:E1633–8. [DOI] [PubMed] [Google Scholar]
- [15].Branco BC, Plurad D, Green DJ, et al. Incidence and clinical predictors for tracheostomy after cervical spinal cord injury: a National Trauma Databank review. J Trauma 2011;70:111–5. [DOI] [PubMed] [Google Scholar]
- [16].Berney S, Bragge P, Granger C, et al. The acute respiratory management of cervical spinal cord injury in the first 6 weeks after injury: a systematic review. Spinal Cord 2011;49:17–29. [DOI] [PubMed] [Google Scholar]
- [17].Tollefsen E, Fondenes O. Respiratory complications associated with spinal cord injury. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke 2012;132:1111–4. [DOI] [PubMed] [Google Scholar]
- [18].Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehab 1994;75:270–5. [DOI] [PubMed] [Google Scholar]
- [19].Liebscher T, Niedeggen A, Estel B, et al. Airway complications in traumatic lower cervical spinal cord injury: a retrospective study. J Spinal Cord Med 2015;38:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- [21].Suberviola B, Gonzalez-Castro A, Llorca J, et al. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury 2008;39:748–52. [DOI] [PubMed] [Google Scholar]
- [22].Hassid VJ, Schinco MA, Tepas JJ, et al. Definitive establishment of airway control is critical for optimal outcome in lower cervical spinal cord injury. J Trauma 2008;65:1328–32. [DOI] [PubMed] [Google Scholar]
- [23].Kornblith LZ, Kutcher ME, Callcut RA, et al. Mechanical ventilation weaning and extubation after spinal cord injury: a Western Trauma Association multicenter study. J Trauma Acute Care Surg 2013;75:1060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seidl RO, Wolf D, Nusser-Muller-Busch R, et al. Airway management in acute tetraplegics: a retrospective study. Eur Spine J 2010;19:1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ouchida J, Yukawa Y, Ito K, et al. Delayed magnetic resonance imaging in patients with cervical spinal cord injury without radiographic abnormality. Spine 2016;41:E981–6. [DOI] [PubMed] [Google Scholar]
- [26].Huang YH, Ou CY. Magnetic resonance imaging predictors for respiratory failure after cervical spinal cord injury. Clin Neurol Neurosurg 2014;126:30–4. [DOI] [PubMed] [Google Scholar]
- [27].D'Souza MM, Choudhary A, Poonia M, et al. Diffusion tensor MR imaging in spinal cord injury. Injury 2017;48:880–4. [DOI] [PubMed] [Google Scholar]
- [28].Battistuzzo CR, Armstrong A, Clark J, et al. Early decompression following cervical spinal cord injury: examining the process of care from accident scene to surgery. J Neurotrauma 2015;33:1161–9. [DOI] [PubMed] [Google Scholar]
- [29].Babu R, Owens TR, Thomas S, et al. Timing of tracheostomy after anterior cervical spine fixation. J Trauma Acute Care Surg 2013;74:961–6. [DOI] [PubMed] [Google Scholar]
- [30].Furlan JC, Craven BC, Massicotte EM, et al. Early versus delayed surgical decompression of spinal cord after traumatic cervical spinal cord injury: a cost-utility analysis. World Neurosurg 2016;88:166–74. [DOI] [PubMed] [Google Scholar]
- [31].Bourassa-Moreau E, Mac-Thiong JM, Ehrmann Feldman D, et al. Complications in acute phase hospitalization of traumatic spinal cord injury: does surgical timing matter? J Trauma Acute Care Surg 2013;74:849–54. [DOI] [PubMed] [Google Scholar]
- [32].Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine 2006;31(11 suppl):S28–35. [DOI] [PubMed] [Google Scholar]
- [33].Jug M, Kejzar N, Vesel M, et al. Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours versus 8 to 24 hours after injury: a single center experience. J Neurotrauma 2015;32:1385–92. [DOI] [PubMed] [Google Scholar]
- [34].Liu Y, Shi CG, Wang XW, et al. Timing of surgical decompression for traumatic cervical spinal cord injury. Int Orthop 2015;39:2457–63. [DOI] [PubMed] [Google Scholar]
- [35].Ropper AE, Neal MT, Theodore N. Acute management of traumatic cervical spinal cord injury. Pract Neurol 2015;15:266–72. [DOI] [PubMed] [Google Scholar]
- [36].Xu ZW, Lun DX. Surgical management of multilevel cervical spinal stenosis and spinal cord injury complicated by cervical spine fracture. J Orthop Surg Res 2014;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med 2007;30:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med 2007;30:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liang HW, Wang YH, Lin YN, et al. Impact of age on the injury pattern and survival of people with cervical cord injuries. Spinal Cord 2001;39:375–80. [DOI] [PubMed] [Google Scholar]
- [40].Winslow C, Bode RK, Felton D, et al. Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest 2002;121:1548–54. [DOI] [PubMed] [Google Scholar]
- [41].Raurich JM, Rialp G, Llompart-Pou JA, et al. Respiratory CO(2) response in acute cervical spinal cord injury (CO(2) response in spinal cord injury). Spinal Cord 2014;52:39–43. [DOI] [PubMed] [Google Scholar]
- [42].Berlowitz DJ, Tamplin J. Respiratory muscle training for cervical spinal cord injury. Cochrane Database Syst Rev 2013;7:CD008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Berney SC, Gordon IR, Opdam HI, et al. A classification and regression tree to assist clinical decision making in airway management for patients with cervical spinal cord injury. Spinal Cord 2011;49:244–50. [DOI] [PubMed] [Google Scholar]


