Abstract
Whether the survival outcome of patients with non-metastatic esophageal squamous cell carcinoma (NM-ESCC) receiving definitive concurrent chemoradiotherapy (CCRT) is better with intensity-modulated radiotherapy (IMRT) or with 3-dimensional conformal radiotherapy (3DCRT) has been debated in the literature. We designed this population-based propensity-score (PS)-matched analysis to address this question. We identified eligible patients diagnosed between 2008 and 2015 from the Taiwan Cancer Registry and constructed a PS-matched cohort (1:1 for IMRT vs 3DCRT) to balance observable potential confounders. We compared the hazard ratio (HR) of death between IMRT and 3DCRT during the entire follow-up period. We also evaluated freedom from local regional recurrence (FFLRR) and esophageal cancer-specific survival (ECSS). Sensitivity analyses (SA) were performed to examine the robustness of our findings. Our study population constituted 558 patients who were well balanced with regard to the measured covariables. The HR of death with IMRT compared to 3DCRT was 0.43 (95% confidence interval 0.35–0.52, P < .001). The results remained significant for FFLRR and ECSS. In SA, our results remained significant when additional covariables were taken into consideration. The survival outcome of patients with NM-ESCC receiving CCRT might be better with IMRT vs 3DCRT. These study results should be interpreted with caution given some possible covariates lacking in the registry. Further studies are needed to clarify this issue.
Keywords: 3-dimensional conformal radiotherapy, concurrent chemoradiotherapy, esophageal squamous cell carcinoma, intensity-modulated radiotherapy
1. Introduction
Esophageal cancer is one the leading causes of cancer death worldwide.[1] Squamous cell carcinoma (SqCC) is the most prevalent type of this cancer, particularly in the West.[1,2] For localized esophageal SqCC, concurrent chemoradiotherapy (CCRT) is the standard of care, whereas radiotherapy (RT) alone has no longer been considered appropriate following the RTOG 85-01 trial.[3–5] Regarding RT for non-metastatic esophageal squamous cell carcinoma (NM-ESCC), National Comprehensive Cancer Network Guidelines for Esophageal and Esophagogastric Junction Cancers (v.1.2017) state that “Intensity-modulated radiotherapy (IMRT) is appropriate in clinical settings where reduction in dose to organs in risk…cannot be achieved by 3D techniques.”[4]
The IMRT is an advanced radiotherapy technology. Cao et al compared IMRT to two-dimensional RT in 37 patients with cervical esophageal cancer receiving definitive RT and found lower late toxicity with IMRT.[6] Retrospective comparative studies had also suggested that the dosimetric advantage of IMRT over 3-dimensional conformal radiotherapy (3DCRT) can translate into improved clinical outcomes.[7] However, as reported in a 2017 systematic review, clinical data for 3DCRT vs IMRT regarding “hard” endpoints such as overall survival (OS) are scarce, especially in the definitive setting.[8–11] Lin et al reported better OS with IMRT compared to 3DCRT for patients with esophageal cancer treated with neoadjuvant CCRT followed by surgery.[9] A small (n = 60) randomized trial was published in Chinese in which the authors found no statistical difference in OS between 3DCRT and IMRT, although it was not specified in the English abstract whether or not the study was limited to a definitive setting (ie, whether neoadjuvant therapy was allowed).[10] In addition, in a retrospective analysis also published in Chinese, the authors found no statistical difference in OS between 3DCRT and IMRT.[11] Whether surgery was allowed or not was not specified in the Chinese abstract. Given the controversy regarding this issue, as illustrated in the research cited above, the aim of the present study was to investigate the survival outcome of patients with NM-ESCC receiving definitive CCRT via either IMRT or 3DCRT in this population-based propensity-score (PS)-matched analysis.
2. Materials and methods
2.1. Study setting and data source
Our study setting was a case/control study using the Taiwan Cancer Registry (TCR) and death registration. The TCR is a high-quality comprehensive cancer registry for the entire population of Taiwan[12] and provides sufficient information regarding individual demographics, stage of disease, tumor histology, and treatment details.
2.2. Study population and study design
Our study flowchart as suggested by the STROBE guidelines[13] is depicted in Figure 1. The study population consisted of patients with NM-ESCC who were diagnosed and received definitive CCRT with external beam RT between 2008 and 2015. We defined CCRT as concurrent systemic therapy during local regional therapy according to data recorded in the TCR. We adopted the date of diagnosis in the cancer registry as the index date and determined the explanatory variable of interest (either IMRT or 3DCRT). We also collected covariables (see Section 2.3) for adjustment of potential non-randomized treatment selection, which was in general at the discretion of the radiation oncologists in charge. We then adopted a PS matching method to construct a matched sample and performed survival analysis to evaluate the effect of IMRT vs 3DCRT.
Figure 1.
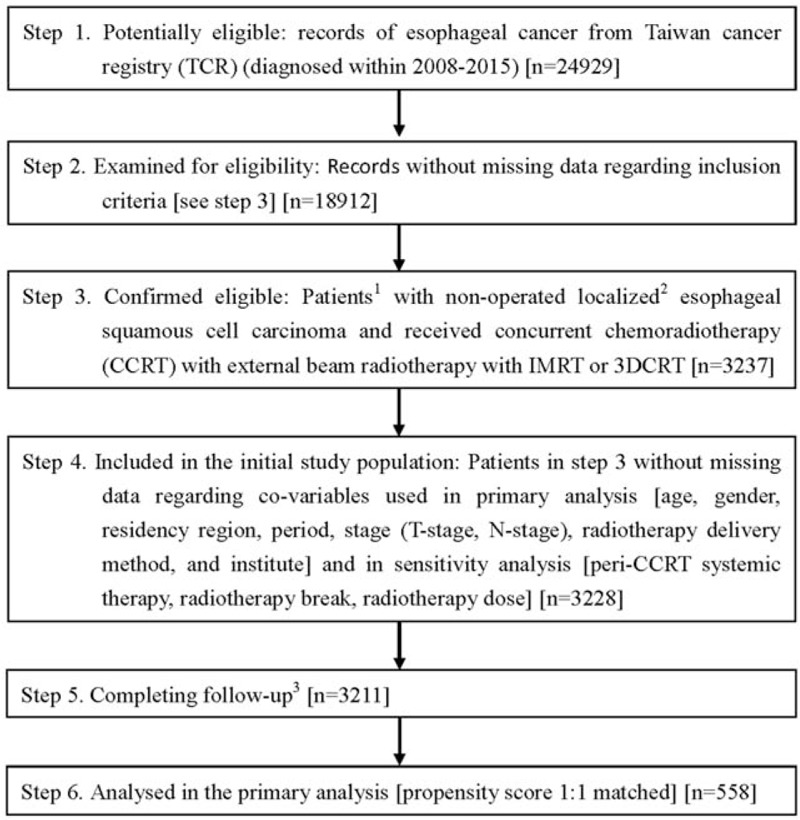
STROBE study flowchart and numbers of individuals at each stage of study. 1 We only included those treated (classes 1–2) at any single institution to ensure data consistency. 2 6th American Joint Committee on Cancer staging clinical stages 2 to 4a (2008–2009) or 7th stages 2 to 3 (2010–2015). 3 Without missing information in TCR and death registry.
2.3. Other explanatory covariables
In this study, we included patient demographics (age, sex, residency region), disease characteristics (clinical T-stage and N-stage), period, institution, and RT delivery (image guided [IG] or not) in our primary analysis. We also considered 3 “variables of ambiguous status” (“perhaps slightly affected by the treatment, but plausibly standing in as a surrogate for an important covariable that was not measured”[14]) in the sensitivity analysis (SA) (see Section 3.3). These 3 variables of ambiguous status were the use of peri-CCRT (ie, induction or consolidative) systemic therapy, RT break, and RT dose. The selection and definition (including grouping of quantitative variables) of these factors were based on our experiences in clinical care and modified from our prior TCR-related studies.[15–20] The tumor/node/metastasis classification was based on 6th edition (2008–2009 cases) or 7th edition (2010–2015 cases). The definitions of our covariables were as follows. Age was classified as ≥65 years old or not. Patient residency region was classified as northern Taiwan or elsewhere. T-stage was classified as T1-T2 or T3-T4. N-stage was classified as positive (N1M0 or N0-1M1a [2008–2009]; N1-N3 [2010–2015]) or negative. Period was classified as 2008 to 2009 vs 2010 to 2011 vs 2012 to 2015. Peri-CCRT systemic therapy was classified as yes or no. External beam RT delivery was classified as IGRT or non-IGRT. The institution was classified as a high-volume institute vs a low-volume institute with the cut-off point roughly in the middle of the study sample. The RT break interval was calculated by the exact RT duration (week) minus the expected RT duration (by 5 fractions per week) and classified as >1 week or ≤1 week.
2.4. Effectiveness assessment in primary analysis
The survival statuses of patients were obtained from the death registry (follow-up until December 31, 2016). We used this information to compare OS of patients between the IMRT and 3DCRT groups as our primary analysis. We also evaluated freedom from local regional recurrence (FFLRR) and esophageal cancer-specific survival (ECSS) according to the TCR and death registry.
2.5. Statistical analysis
Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC) and STATA 12 (StataCorp LP, College Station, TX). We used logistic regression models with the above covariables to obtain the estimated PS values. We then constructed matched groups (IMRT vs 3DCRT) based on the logit of PS via 1:1 matching (caliper width = 0.0001) and exact matching on period (2008–2009/2010–2012/2013–2015). We used standardized difference to assess the differences in covariates between the 2 groups as suggested in the recent review papers.[21,22] We compared the hazard ratio (HR) of events between IMRT and 3DCRT groups using Cox proportional hazards models. The confidence interval (CI) was estimated via a robust variance estimator.[23] We calculated the E-value to evaluate the robustness of our primary analysis regarding potential unmeasured confounder(s) as suggested in the literature.[24] We performed additional SA to further examine the robustness of our findings. In the first SA, we included the 3 variables of ambiguous status as mentioned above in our analysis and constructed another PS-matched population for analysis. Regarding tumor location, definitive CCRT rather than surgery was usually preferred and a higher dose (60–70 Gy rather than around 50 Gy) was often favored for cervical NM-ESCC in contrast to NM-ESCC at other locations.[4,25] Therefore, the benefit of IMRT might be different between cervical esophageal cancer and other sites. Accordingly, in the second SA, we constructed another study population to control for tumor location (cervical or not) in addition to those used in our primary analysis. This study was approved by Research Ethics Committee, National Health Research Institutes (EC1041006-E).
3. Results
3.1. Identification of study population used in primary analysis
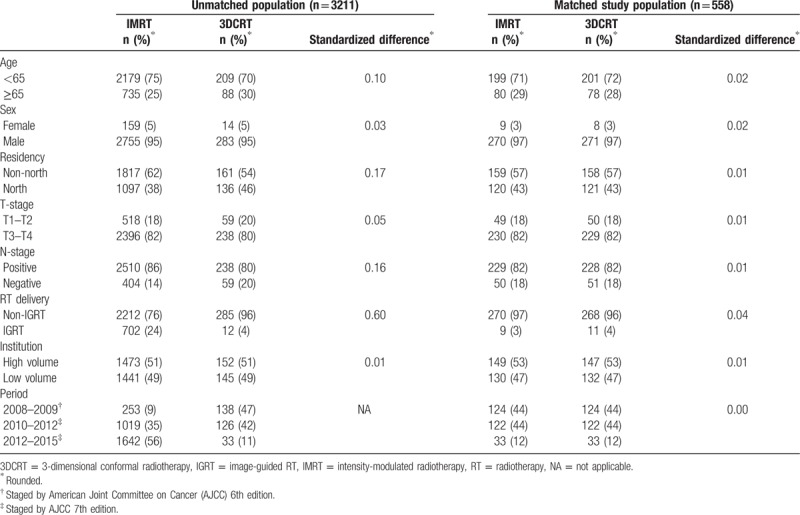
As shown in Figure 1, 3228 patients with cancer who received CCRT with external beam RT, with groups receiving either IMRT or 3DCRT, were identified as the initial study population. After exclusion of missing data during follow-up, and using the PS matching method, the final study population used in the primary analysis included 558 patients. The patient characteristics are described in Table 1. Some imbalance in covariables was observed before matching, but good balance in covariables and small standardized differences (<0.25) were seen for all covariables after matching.[21]
Table 1.
Characteristics of unmatched and matched study population in primary analysis.
3.2. Primary analysis
After a median follow-up period of 17 months (range 1–107), death was observed for 153 patients in the IMRT group and 239 in the 3DCRT group. The HR of death when IMRT was compared to 3DCRT was 0.43 (95% CI 0.35–0.52, P < .001]. The observed HR of 0.43 for OS could be explained away by an unmeasured confounder that was associated with both selection of IMRT/3DCRT and survival/death by a risk ratio of 2.97-fold each (ie, E-value was 2.97), but weaker confounding could not explain away this finding. The 5-year OS rate was 44% for IMRT vs 15% for 3DCRT. The Kaplan–Meier survival curve for OS is shown in Figure 2. IMRT was also associated with better FFLRR (HR 0.53, 95% CI 0.43–0.65, P < .001) and ECSS (HR 0.37, 95% CI 0.29–0.46, P < .001).
Figure 2.
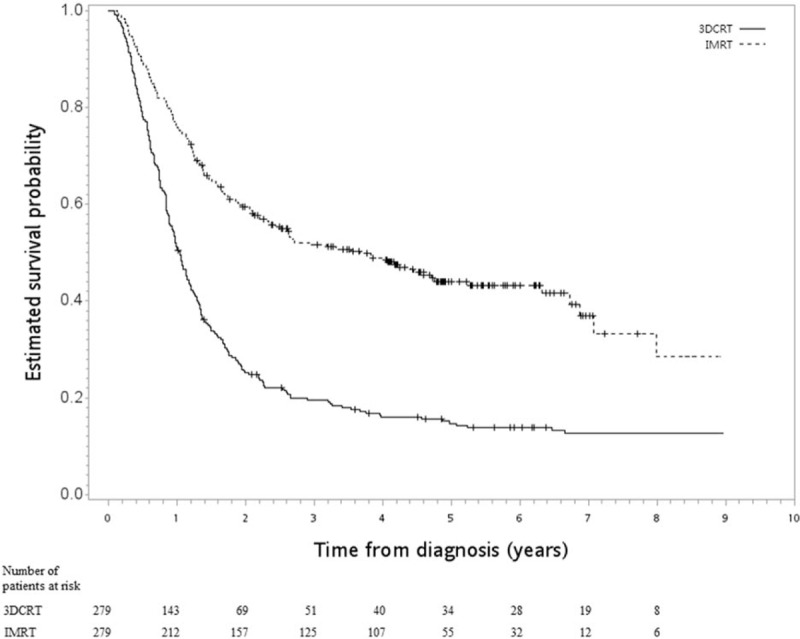
Kaplan–Meier survival curve (in years) in primary analysis.
3.3. Sensitivity analysis
In the first SA, good balance in covariables and small standardized differences (<0.25) were still achieved for all covariables after matching (n = 332) (Table 2). The HR of death when IMRT was compared to 3DCRT was 0.76 (95% CI 0.59–0.96, P = .02). The Kaplan–Meier survival curve for OS is shown in Figure 3. In the second SA, when tumor location was also considered, good balance in covariables and small standardized differences (<0.25) were still achieved for all covariables after matching (n = 460) (Table 3). The HR of death when IMRT was compared to 3DCRT was 0.42 (95% CI 0.34–0.53, P < .001). The Kaplan–Meier survival curve for OS is shown in Figure 4.
Table 2.
Characteristics of the matched study population in the 1st sensitivity analyses.
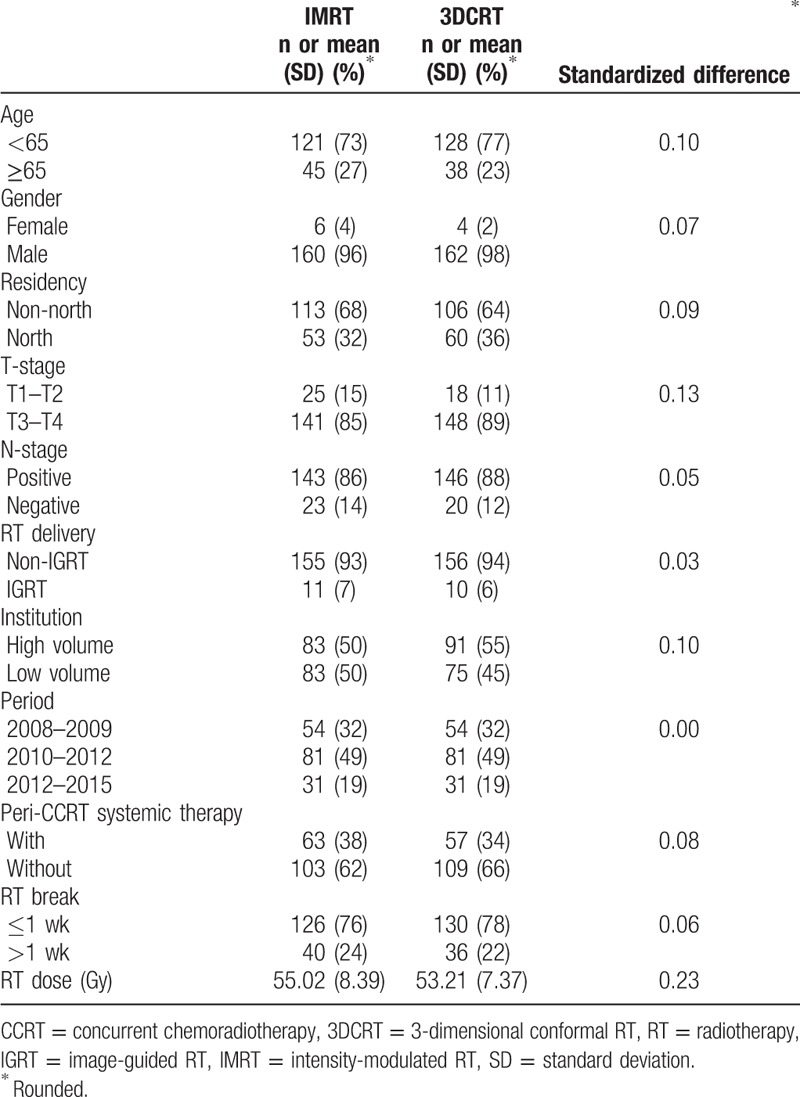
Figure 3.
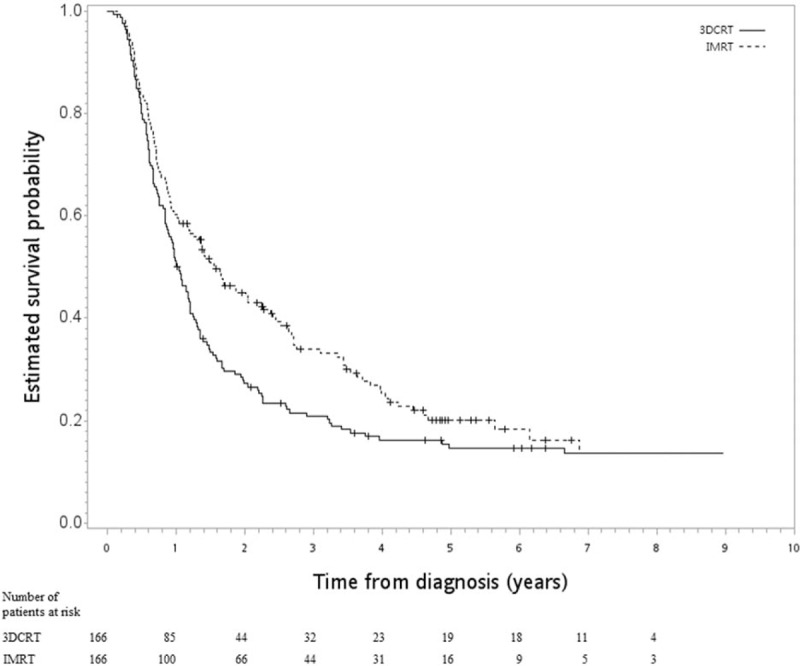
Kaplan–Meier survival curve (in years) in 1st SA.
Table 3.
Characteristics of matched study population in the 2nd sensitivity analyses.
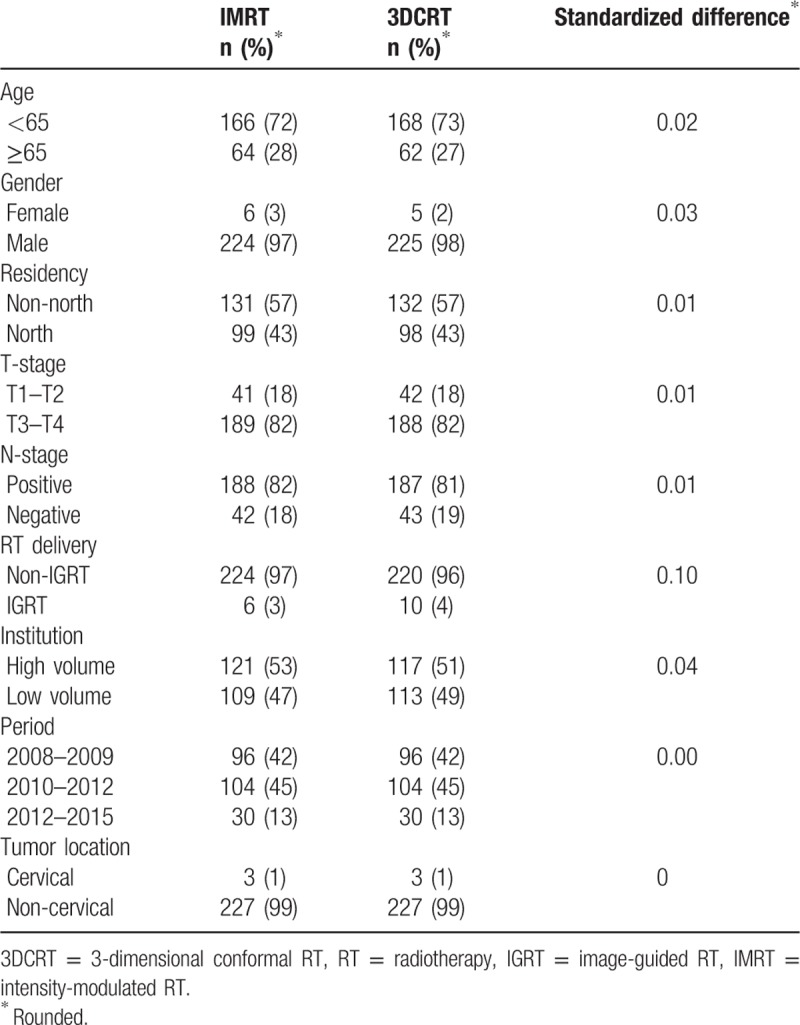
Figure 4.
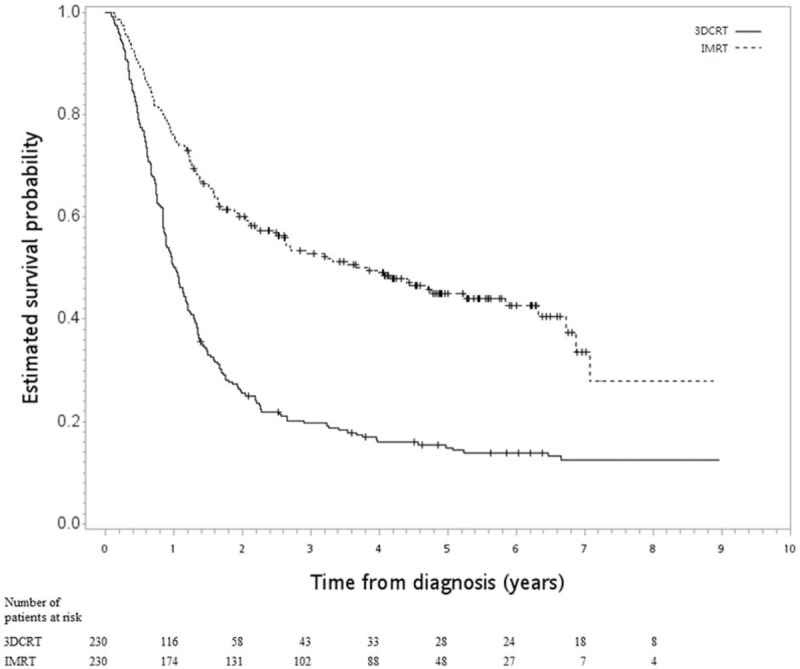
Kaplan–Meier survival curve (in years) in 2nd SA.
4. Discussion
In this population-based PS-matched analysis, we found that the survival outcome of patients with NM-ESCC receiving CCRT was better with IMRT than with 3DCRT. This is the first populated-based evidence regarding survival outcome, to our knowledge.
In addition to the studies included in the recent systemic review,[8] we had also noted 4 single institute studies reporting on the effect of IMRT vs 3D for patients with esophageal cancer receiving definitive CCRT published in 2016 to 2017.[26–29] He et al reported a greater survival probability when IMRT was compared to 3DCRT.[26] Similarly, McDowell et al reported a trend (P = .06) in favor of IMRT when compared to 3DCRT.[27] Haefner et al reported lower local recurrence and an insignificant survival difference (3DCRT vs IMRT: median 18 vs 42 months, P = .2).[28] Finally, Ito et al reported a 3-year OS of 82% (IMRT) vs 57% (3DCRT) among 80 patients with cervical esophageal cancer.[29] Our results were consistent with the findings of these single institution studies in that IMRT was associated with numerically better survival.[26–29] The superiority of IMRT had also been reported in the neoadjuvant setting[9] and in another population-based study in which surgery and chemotherapy were considered as covariables.[30] The survival in our IMRT group was also approaching the results observed in a single arm study.[31] Given the potential benefit of IMRT as seen in other disease sites,[32] our results seem reasonable. However, there were several limitations of our study. Firstly, as discussed above, our findings could result from unmeasured confounding factors, although we used PS matching to balance observed confounders. However, there remain possible unmeasured confounder(s) (such as performance status, body weight loss, or comorbidity status, which were data not available to us) to bias our results. Therefore, we reported the E-value to examine this “no unmeasured confounder assumption” as suggested in the literature.[24] Secondly, the lack of treatment details such as chemotherapy drugs, prophylactic nodal irradiation, or involved-field radiotherapy, owing to data inavailability, could make it difficult to compare our results with the findings of other reports. However, the impact of this unmeasured covariate (prophylactic nodal irradiation or involved-field radiotherapy) might be less significant because similar outcomes had been reported in a previous randomized trial.[33] Thirdly, we did not analyze dosimetry details or other outcomes such as toxicity or quality of life owing to data limitations.
In our mind, the interpretation of our findings (improved FFLRR, ECSS, and OS by IMRT) should be cautious given the non-randomized nature of our study and the possible unmeasured confounder(s). On the one hand, IMRT has potential dosimetric benefit that might translate into clinical benefit, as stated in the previous review papers.[7,8] On the other hand, as seen in our SA-1, the inclusion of variables of ambiguous status (“perhaps slightly affected by the treatment, but plausibly standing in as a surrogate for an important covariable that was not measured”[14]) reduced the HR (ie, OS benefit) to 0.76 (95% CI 0.59–0.96) from 0.43 (95% CI 0.35–0.52) in the primary analysis. The survival curve also appeared much closer for IMRT vs 3DCRT (Fig. 3).
Therefore, the implication of our study is not that we confirmed the superiority of IMRT vs 3DCRT, owing to the above-mentioned study limitations and controversy seen with the use of IMRT in other disease sites, such as in lung cancer.[16] Rather, our study demonstrated the potential superiority of IMRT as further studies are awaited (hopefully phase 3 in the future) to clarify this issue. However, when we searched www.clinicaltrials.gov using “esophageal cancer | Interventional Studies | intensity modulated radiotherapy | Phase 3” on January 23, 2018, we did not find trials designed to compare IMRT vs 3DCRT. In addition, the implications for cervical NM-ESCC should be cautious given the small number of cases (1% in SA-2).
In conclusion, we provided the first population-based evidence that the survival outcome of patients with NM-ESCC receiving definitive CCRT might be better with IMRT than with 3DCRT. These results should be interpreted with caution given some possible covariates lacking in the data registry. Further studies are needed to clarify this issue.
Acknowledgments
The authors thank “Wolters Kluwer Author Services” for editorial assistance. The corresponding author thanks Dr Ya-Chen Tina Shih for her mentoring in Health Services Research.
Author contributions
Conceptualization: Chia-Chin Li, Chih-Yi Chen, Chun-Ru Chien.
Formal analysis: Chia-Chin Li, Chun-Ru Chien.
Investigation: Chih-Yi Chen, Chun-Ru Chien.
Methodology: Chun-Ru Chien.
Writing – original draft: Chun-Ru Chien.
Writing – review & editing: Chia-Chin Li, Chih-Yi Chen, Chun-Ru Chien.
Footnotes
Abbreviations: CCRT = concurrent chemoradiotherapy, 3DCRT = 3-dimensional conformal radiotherapy, ECSS = esophageal cancer specific survival, FFLRR = freedom from local regional recurrence, HR = hazard ratio, 95% CI = 95% confidence interval, IG = image guided, IMRT = Intensity-modulated radiotherapy, NM-ESCC = non-metastatic esophageal squamous cell carcinoma, OS = overall survival, PS = propensity score, RT = radiotherapy, SA = sensitivity analyses, SqCC = squamous cell carcinoma, TCR = Taiwan Cancer Registry.
This work is supported by Health Promotion Administration, Ministry of Health and Welfare, Taiwan (ROC). Sources of funding are the tobacco control and health care funds.
Chia-Chin Li and Chih-Yi Chen contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400–12. [DOI] [PubMed] [Google Scholar]
- [2].Chien CR, Lin CY, Chen CY. Re: Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2009;101:1428author reply 1429. [DOI] [PubMed] [Google Scholar]
- [3].National Comprehensive Cancer Network Guidelines for Esophageal Cancers, version 1.2008. Available at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf [free registration required]. Accessed July 2, 2008. [Google Scholar]
- [4].National Comprehensive Cancer Network Guidelines for Esophageal and Esophagogastric Junction Cancers, version 1.2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf [free registration required]. Accessed July 19, 2017. [Google Scholar]
- [5].Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8. [DOI] [PubMed] [Google Scholar]
- [6].Cao C, Luo J, Gao L, et al. Definitive intensity-modulated radiotherapy compared with definitive conventional radiotherapy in cervical oesophageal squamous cell carcinoma. Radiol Med 2015;120:603–10. [DOI] [PubMed] [Google Scholar]
- [7].Xi M, Lin SH. Recent advances in intensity modulated radiotherapy and proton therapy for esophageal cancer. Expert Rev Anticancer Ther 2017;17:635–46. [DOI] [PubMed] [Google Scholar]
- [8].Xu D, Li G, Li H, et al. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin XD, Shi XY, Zhou TC, et al. Intensity-modulated or 3-D conformal radiotherapy combined with chemotherapy with docetaxel and cisplatin for locally advanced esophageal carcinoma. Nan Fang Yi Ke Da Xue Bao 2011;31:1264–7. [PubMed] [Google Scholar]
- [11].Qiu R, Wang J, Wang YX, et al. Clinical contrast study of esophageal carcinoma treated with three-dimensional conformal radiotherapy and intensity modulated radiation therapy. Chin J Cancer Prevent Treat 2012;68–71. http://caod.oriprobe.com/articles/29156242/Clinical_contrast_study_of_esophageal_carcinoma_tr.htm [Google Scholar]
- [12].Chiang CJ, You SL, Chen CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol 2015;45:291–6. [DOI] [PubMed] [Google Scholar]
- [13].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- [14].Rosenbaum PR. “Section 18.2: Are analytic adjustments feasible?” and “Solutions to common problems”. In: Rosenbaum PR. Design of Observational Studies. New York: Springer Series in Statistics: Springer. 2010:317-21, 355-357. [Google Scholar]
- [15].Chien CR, Hsia TC, Chen CY. Cost-effectiveness of chemotherapy combined with thoracic radiotherapy versus chemotherapy alone for limited stage small cell lung cancer: a population-based propensity-score matched analysis. Thorac Cancer 2014;5:530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hsia TC, Tu CY, Chen HJ, et al. A population-based study of primary chemoradiotherapy in clinical stage III non-small cell lung cancer: intensity-modulated radiotherapy versus 3D conformal radiotherapy. Anticancer Res 2014;34:5175–80. [PubMed] [Google Scholar]
- [17].Hsia TC, Tu CY, Fang HY, et al. Cost and effectiveness of image-guided radiotherapy for non-operated localized lung cancer: a population-based propensity score-matched analysis. J Thorac Dis 2015;7:1643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin CY, Fang HY, Feng CL, et al. Cost-effectiveness of neoadjuvant concurrent chemoradiotherapy versus esophagectomy for locally advanced esophageal squamous cell carcinoma: A population-based matched case-control study. Thorac Cancer 2016;7:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen CY, Li CC, Chien CR. Does higher radiation dose lead to better outcome for non-operated localized esophageal squamous cell carcinoma patients who received concurrent chemoradiotherapy? A population based propensity-score matched analysis. Radiother Oncol 2016;120:136–9. [DOI] [PubMed] [Google Scholar]
- [20].Li CC, Chen CY, Chien CR. Comparative effectiveness of image-guided radiotherapy for non-operated localized esophageal squamous cell carcinoma patients receiving concurrent chemoradiotherapy: a population-based propensity score matched analysis. Oncotarget 2016;7:71548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res 2014;49:1701–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ali MS, Groenwold RH, Belitser SV, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol 2015;68:112–21. [DOI] [PubMed] [Google Scholar]
- [23].Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- [25].Hoeben A, Polak J, Van De Voorde L, et al. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol 2016;27:1664–74. [DOI] [PubMed] [Google Scholar]
- [26].He L, Chapple A, Liao Z, et al. Bayesian regression analyses of radiation modality effects on pericardial and pleural effusion and survival in esophageal cancer. Radiother Oncol 2016;121:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McDowell LJ, Huang SH, Xu W, et al. Effect of intensity modulated radiation therapy with concurrent chemotherapy on survival for patients with cervical esophageal carcinoma. Int J Radiat Oncol Biol Phys 2017;98:186–95. [DOI] [PubMed] [Google Scholar]
- [28].Haefner MF, Lang K, Verma V, et al. Intensity-modulated versus 3-dimensional conformal radiotherapy in the definitive treatment of esophageal cancer: comparison of outcomes and acute toxicity. Radiat Oncol 2017;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ito M, Kodaira T, Tachibana H, et al. Clinical results of definitive chemoradiotherapy for cervical esophageal cancer: comparison of failure pattern and toxicities between intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy. Head Neck 2017;39:2406–15. [DOI] [PubMed] [Google Scholar]
- [30].Lin SH, Zhang N, Godby J, et al. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer 2016;122:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roeder F, Nicolay NH, Nguyen T, et al. Intensity modulated radiotherapy (IMRT) with concurrent chemotherapy as definitive treatment of locally advanced esophageal cancer. Radiat Oncol 2014;9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].De Neve W, De Gersem W, Madani I. Rational use of intensity-modulated radiation therapy: the importance of clinical outcome. Semin Radiat Oncol 2012;22:40–9. [DOI] [PubMed] [Google Scholar]
- [33].Ma JB, Song YP, Yu JM, et al. Feasibility of involved-field conformal radiotherapy for cervical and upper-thoracic esophageal cancer. Onkologie 2011;34:599–604. [DOI] [PubMed] [Google Scholar]


