Table 2.
Ni/Photoredox Dual Catalytic C(sp2)-C(sp3) Cross-Coupling on-DNAa
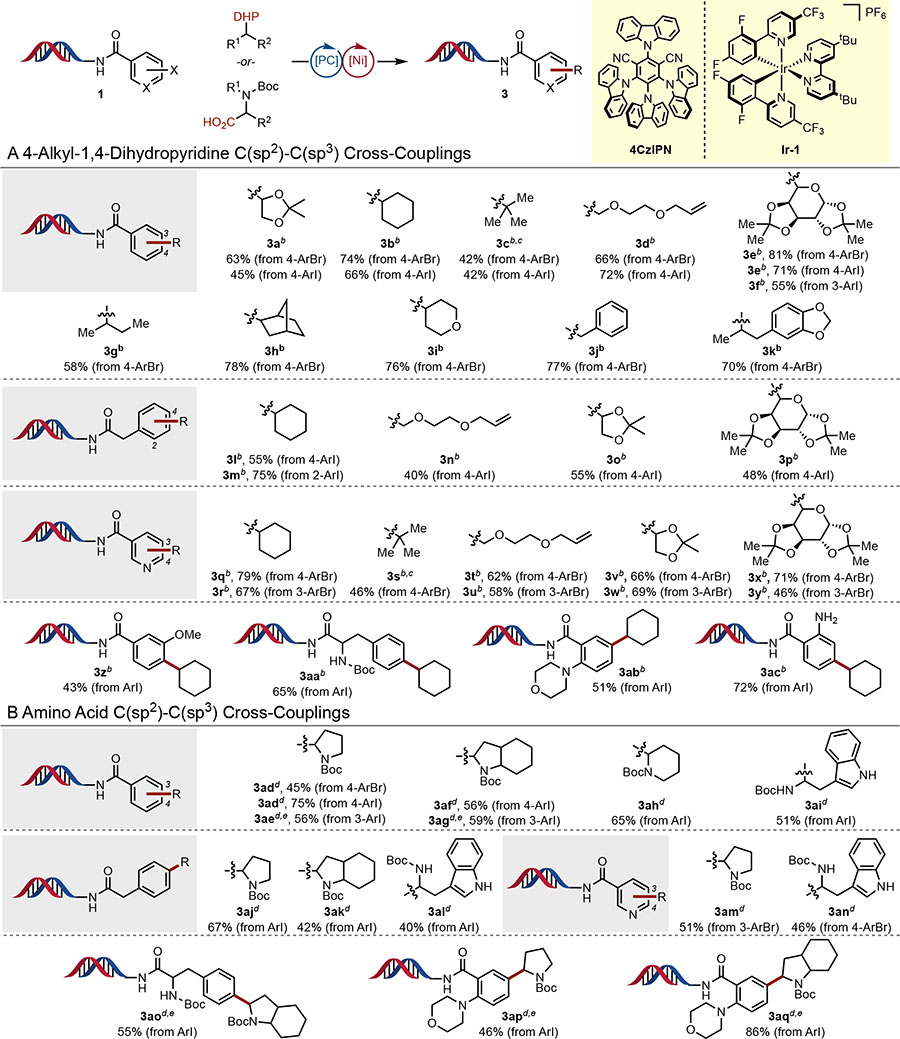 |
All values indicate conversion to the indicated product as determined by LC/MS.
Reaction conditions: DHP (250 equiv, 6.25 μmol), 4CzIPN (50 mol %, 12.5 nmol), Ni(TMHD)2 (2.0 equiv, 50 nmol), aryl halide (25 nmol, 1.0 equiv), 80:20 DMSO/H2O (1 mM), 45 min, irradiating with blue LED (30 W).
Using 4-(tert-butyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarbonitrile.
Reaction conditions: amino acid (250 equiv, 6.25 μmol), Ir-1 (50 mol %, 12.5 nmol), Ni(TMHD)2 (2.0 equiv, 50 nmol), aryl halide (25 nmol, 1.0 equiv), TMG (700 equiv, 17.5 μmol), MOPS pH 8 buffer (25 mM), 77:23 DMSO/H2O (1 mM), 10 min, irradiating with blue LED (30 W).
Using TMG (990 equiv, 24.75 μmol) and 30 min reaction time. TMG = 1,1,3,3-tetramethylguanidine. MOPS = 3-(N-morpholino)propanesulfonic acid. See Supporting Information for additional details.
