Abstract
Objective
Vascular smooth muscle cells (VSMCs) phenotype modulation is critical for the resolution of vascular injury. Genetic and pharmacological inhibition of phosphoinositide 3-kinase γ (PI3Kγ) exerts anti-inflammatory and protective effects in multiple cardiovascular diseases. This study investigated the role of PI3Kγ and its downstream effector molecules in the regulation of VSMC phenotypic modulation and neointimal formation in response to vascular injury.
Approach and Results
Increased expression of PI3Kγ was found in injured vessel wall as well in cultured, serum-activated wildtype VSMCs, accompanied by a reduction in the expression of calponin and SM22α, two differentiation markers of VSMCs. However, the injury-induced downregulation of calponin and SM22α were profoundly attenuated in PI3Kγ−/− mice. Pharmacological inhibition and shRNA knockdown of PI3Kγ (PI3Kγ-KD) markedly attenuated yes-associated protein (YAP) expression and cyclic AMP-response element binding protein (CREB) activation but improved the downregulation of differentiation genes in cultured VSMCs accompanied by reduced cell proliferation and migration. Mechanistically, activated CREB upregulated YAP transcriptional expression through binding to its promoter. Ectopic expression of YAP strikingly repressed the expression of differentiation genes even in PI3Kγ-KD VSMCs. Moreover, established carotid artery ligation and chimeric mice models demonstrate that deletion of PI3Kγ in naïve PI3Kγ−/− mice as well as in chimeric mice lacking PI3Kγ either in bone marrow or vascular wall significantly reduced neointimal formation following injury.
Conclusions
PI3Kγ controls phenotypic modulation of VSMCs by regulating transcription factor CREB activation and YAP expression. Modulating PI3Kγ signaling on local vascular wall may represent a new therapeutic approach to treat proliferative vascular disease.
Keywords: PI3Kγ, vascular smooth muscle cells, YAP, phenotypic modulation, neointimal formation
INTRODUCTION
Vascular smooth muscle cells (VSMCs) are the fundamental cellular components of the blood vessel wall that primarily govern structural integrity and regulate vascular tone. In the medial layer of normal mature arteries, VSMCs reside in a differentiated quiescent state with very low synthetic activity and proliferation potential.1 However, unlike many terminally differentiated cells, VSMCs possewss remarkable plasticity. Thus, in response to vascular injury, VSMCs undergo a transition in phenotype from a contractile, differentiated state to a synthetic, dedifferentiated phenotype, which is characterized by decreased expression of VSMC contractile marker genes and increased proliferation, migration, and matrix synthesis, subsequently leading to the development of vascular neointimal lesions.1,2 This response is an initial and central event in the process of vascular wound repair following arterial injury. However, extensive neointimal formation leads to luminal narrow or even occlusion of the diseased vessel, resulting in ischemic cardiovascular diseases. It is well established that VSMC phenotypic modulation is a common fundamental mechanism of a variety of vascular proliferative diseases such as atherosclerosis, restenosis after angioplasty, transplant arteriosclerosis, and pulmonary hypertension.3,4 Therefore, elucidation of the molecular mechanisms underlying VSMC phenotypic modulation is crucial for developing effective therapeutic approach to against these vascular diseases.
There is mounting evidence that expression of most VSMC marker genes dependents on a CArG element located in their promoter region, which serves as the binding site for serum response factor (SRF). The SRF-CArG association functions as an integrator of cellular signals that control VSMC phenotypes.3,5 Recently, Myocardin is proved to be a highly potent transcription coactivator of SRF that selectively drives expression of CArG-dependent VSMC marker genes by forming a transcriptional complex with SRF.6 Moreover, myocardin has also been shown to be a key downstream mediator of diverse signaling pathways governing VSMC phenotypic modulation in response to various environmental cues.5,6 The Hippo pathway was initially described in Drosoplisa about two decades ago, and recent studies have identified the conserved pathway as a crucial regulator of organ size control, tissue homeostasis and tumorigenesis in mammals.7 The kinase cascade of mammalian sterile 20-like 1/2 (Mst1/2) and large tumor suppressor homolog 1/2 (Lats1/2) are referred to as the core components of the mammalian Hippo pathway. Mst1/2 forms a complex with a scaffold protein Salvador (Sav1) and subsequently phosphorylates the Lats1/2 kinase.8 Yes-associated protein (YAP) / transcriptional co-activator with PDZ-binding motif (TAZ), a key downstream effector of Hippo signaling, is phosphorylated and inhibited by activated Lats1/2 kinase that results in cytoplasmic translocation.8 In mammalian cells, YAP mainly interacts with TEAD family transcript factors to regulate gene expression that contributes to cell proliferation.9 There is also evidence that YAP can physically interact with Myocardin and disrupt the formation of Myocardin/SRF complex,10,11 thus suggesting YAP as a critical molecular for VSMC phenotypic modulation.
Phosphatidylinositol 3-kinases (PI3Ks) are a conserved family of lipid kinases that coordinate diverse intracellular signaling pathways and regulate a wide variety of cellular functions by generating the lipid second messenger phosphatidylinositol-3,4,5- trisphosphate.12,13 PI3Ks are divided into three classes based on their substrate specificity. Of these, class I PI3Ks are the most extensively studied and comprise the IA and IB subclasses. PI3Kγ, the unique member of the class IB, is a heterodimer composed of a catalytic subunit p110γ and a regulatory subunit p101 or p84/87.13 Recently, evidence has emerged that p110γ associates with p101 or p87/p84 at the plasma membrane, though which PI3K p110γ can be activated by Gβγ.13,14 PI3Kγ is abundantly expressed in hematopoietic cells and has emerged as a key regulator of a wide range of inflammatory processes, including leukocyte development, recruitment and activation.15 Recently, PI3Kγ has also been found in the cardiovascular system, including smooth muscle cells, endothelial cells and cardiomyocytes. Several lines of evidence have revealed critical roles for PI3Kγ in modulating VSMC migration and contractility that contribute to the progression of neointimal formation and vascular remodeling.16–18 However, the precise contribution of PI3Kγ in VSMCs to neointimal formation and the underlying mechanisms remain poorly understood.
In this study, we provide evidence that PI3Kγ is critically involved in controlling VSMC phenotypic modulation during neointimal formation in response to vascular injury. Vascular injury in vivo and serum in vitro upregulate the expression of PI3Kγ and induce its activation in VSMCs, leading to the downregulation of VSMC-specific markers, accompanied by enhanced VSMC proliferation and migration. Furthermore, cyclic AMP-response element binding protein (CREB)-mediated YAP upregulation is responsible for PI3Kγ/Akt signaling-induced phenotypic modulation of VSMCs. More importantly, using a well-established carotid artery injury model, our results indicate a potent protective effect by PI3Kγ deletion in local vascular cells on neointimal formation and restenosis. These data define a crucial role of PI3Kγ in regulating VSMC phenotypic modulation in response to injury and suggest that local vascular cell-derived PI3Kγ may represent a promising therapeutic target to prevent neointimal formation and restenosis after vascular intervensions.
Materials and Methods
The authors declare that all supporting data are available within the article (and in online-only Data Supplement).
Animals
Male C57BL/6J mice (wild type, WT) were obtained from The Jackson Laboratory (Bar Harbor, ME). PI3K-p110γ knockout (PI3Kγ–/–) mice (backcrossed onto a C57BL/6J background for > 10 generations) were generated,19 and kindly provided by Josef M. Penninger (Institute for Molecular Biotechnology of the Austrian Academy of Sciences, Vienna, Austria). These mice were maintained at the LSU Health Sciences Center-Shreveport (Shreveport, LA) with a specific pathogen-free environment. Only male mice (8–10 weeks old) were used in our study in view of the variability of estrogen levels in female mice, and the inhibition of estrogen in neointimal formation after vascular injury.20,21 All animal protocols were approved by the Institutional Animal Care and Use Committee.
Reagents and antibodies
Reagents and antibodies are listed in the Major Resources Table in the online-only Data Supplement.
Cell culture and treatment
Primary VSMCs were prepared from the thoracic aorta of male C57BL/6J mice using an explant method as previously described.22 For intervention experiments, PI3Kγ specific inhibitor AS605240 (1 µM) was reported to selectively inhibited PI3Kγ enzymatic activity,23 thus, quiescent cells were pretreated with AS605240 (1 µM) for 60 minutes prior to FBS stimulation.
For PI3Kγ, CREB and YAP knockdown, recombinant lentiviruses respectively expressing the short hairpin RNA (shRNA) targeting Pik3cg (PI3K p110γ), CREB and YAP were prepared and used to infect VSMCs as previously described.24 Detailed information is provided in the online-only Data Supplement.
For YAP and CREB ectopic expression, detailed information is provided in the online-only Data Supplement.
Western blotting
Whole cell lysates were prepared with RIPA buffer containing protease inhibitors and quantified using the Bardford Protein Assay (Bio-Rad). Detailed information is provided in the online-only Data Supplement.
Quantitative reverse transcription–polymerase chain reaction (qRT-PCR)
Quantitative RT-PCR was performed as previously described.22 Detailed information is provided in the online-only Data Supplement.
Luciferase activity assay
The luciferase reporter constructs containing YAP promoter sequence (wild type, WT) or mutation sequence (MUT1 and MUT2) were cotransfected with pcCREB plasmid or CREB shRNA or corresponding control vectors as well as the renilla luciferase reporter vector containing thymidine kinase promoter into cultured VSMCs. Luciferase activities were analyzed using a dual-luciferase reporter kit (Promega).
Chromatin Immunoprecipitation (CHIP)
CHIP was carried out using SimpleCHIP ® Plus Sonication Chromatin IP kit (Cell Signaling Technology) according to the manufacturer’s protocol. Additional information is provided in the online-only Data Supplement.
Cell proliferation and migration assays
Cell proliferation was evaluated by Cell Counting Kit-8 (CCK-8) assays and BrdU incorporation assays as previously described.25 VSMC migration was evaluated by wound-healing and Transwell migration assays as previously described.25 Detailed information is provided in the online-only Data Supplement.
Bone marrow transplantation
Bone marrow was obtained from femurs and tibias of 8- to 10-week-old WT and PI3Kγ–/– mice and stored on ice. Recipient mice were lethally irradiated with 2 × 525 rads (3 hours apart) of whole-body irradiation and then received 2 × 106 bone marrow cells by tail vein injection. 8 weeks later, whole blood was collected from chimeric mice and successful BM reconstitution was confirmed using flow cytometry as previously described.26
Carotid ligation model
Carotid artery ligation was performed to induce flow-restriction vascular injury. Briefly, Mice were anesthetized with intraperitoneal administration of ketamine and xylazine. The left common carotid artery was dissected and ligated with a silk suture proximal to the carotid bifurcation. All animals recovered and showed no symptoms of stroke. Mice were sacrificed at the indicated time points after injury. After in situ cardiac perfusion with 4% paraformaldehyde (PFA) or saline, the ligated left and the uninjured right carotid arteries were harvested for histological analysis.
At the indicated time points after injury, mice were euthanized and perfused with normal saline followed by 4% paraformaldehyde (PFA). The femoral arteries were then harvested and post-fixed with 4% PFA overnight.
Histology and morphometric analysis
The arterial segments were dehydrated in ethanol and xylene and embedded in paraffin. Serial cross-sections (5 µm thick) were cut proximal to the carotid ligation site and 7 serial cross-sections (120 µm apart) were selected and stained with Verhoeff’s Elastic stain. Morphometric analysis was performed by two independent investigators blinded to the experimental design using Image-Pro Plus software (Media Cybernetics). We measured the lumen area, intimal area, medial area, and total vessel area at each level as we previously described.22 The mean value of intimal area, intima/media ratio, and lumen stenosis ratio was calculated.
Immunostaining
Immunohistochemistry and Immunofluorescence staining were performed on paraffin-embedded sections using the avidin-biotin-peroxidase complex method (Vector Laboratories). Detailed information is provided in the online-only Data Supplement.
Statistical analysis
Each cell experiment was performed at least three times. All results are expressed as the mean ± SEM. Shapiro-Wilk test and Levene test was respectively applied to assess data distribution and equal variance analysis. For data with normal distribution, Statistical differences between 2 independent groups were analyzed with unpaired 2-tailed Student’s t test. Mann-Whitney U test was used for nonparametric data. Multiple group comparisons were conducted with 1- or 2-way ANOVA followed by Bonferroni post hoc test. A P value less than 0.05 was considered statistically significant.
Results
Vascular injury induces PI3Kγ upregulation and activates PI3Kγ/Akt signaling pathway in VSMCs.
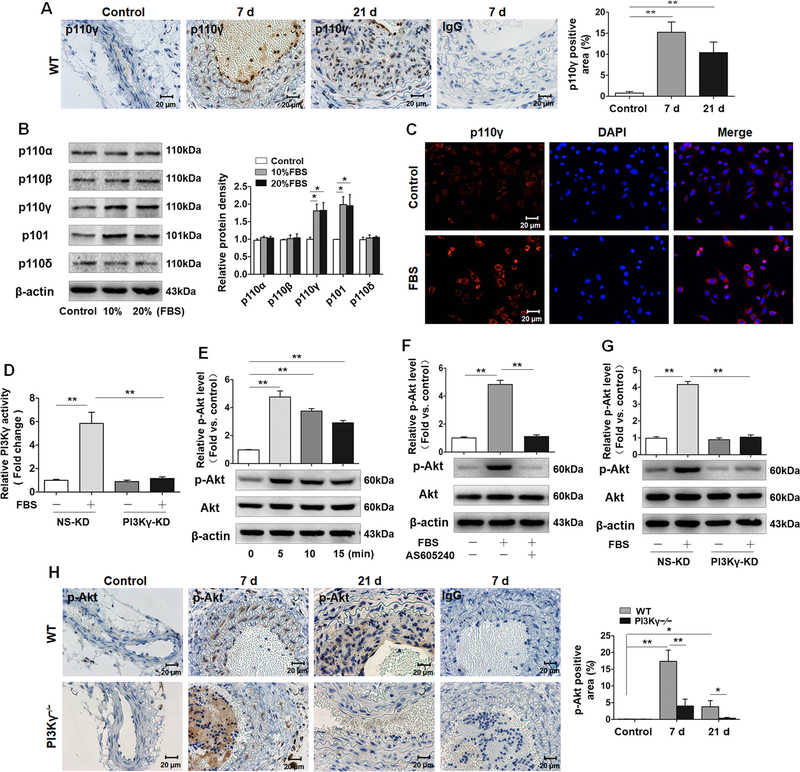
The expression of PI3Kγ has not been studied either in injured arteries or cultured VSMCs. Firstly, we used immunohistochemistry to determine PI3K p110γ expression in the carotid artery wall. Minimal p110γ signals were detected in the uninjured carotid arteries. However, strong p110γ staining signals were noted predominantly in the medial layer of ligated carotid arteries at 7 days and a large number of p110γ-positive cells were observed in the medial and intimal region of the injured carotid arteries at 21 days (Figure 1A). These results indicate that PI3Kγ is expressed and unregulated in arterial VSMCs in response to vascular injury.
Figure 1. Vascular injury induces PI3Kγ upregulation and activates PI3Kγ/Akt signaling pathway in VSMCs.
A, Representative cross sections of ligated carotid arteries from WT (7 and 21 days) immunostained for p110γ (n=8 mice per group) (left panel). IgG acted as the isotype control. The occluded lesion in lumen of ligated artery at 7d is a thrombus. Bar graph (right panel) shows the percentage of p110γ-stained area to medial area. Scale bars: 20 μm. B, Western blotting analysis of p110α, p110β, p110γ, p110δ, and p101 in VSMCs stimulated with 10% and 20% Fetal Bovine Serum (FBS) for 24 h (left panel) (n=6). β-actin acted as loading control. Bar graph (right panel) shows the densitometric analysis. C, Immunofluorescence staining of p110γ (red) in VSMCs stimulated with 10% FBS for 24 h. Cell nuclei were stained with hoechst (blue). Scale bar: 20 μm. D, VSMCs were transfected with PI3Kγ shRNA (PI3Kγ-KD) and corresponding negative control (NS-KD), followed by 10% FBS stimulation for 5 min, and then lysed, immunoprecipitated with PI3K p110γ antibody. PI3Kγ activity was evaluated by measuring the productivity of PIP3 by performing ELISA. E, Western blotting analysis of p-Akt (ser473) and Total Akt in VSMCs stimulated with 10% FBS for indicated time (lower panel). F, Western blotting analysis of p-Akt (ser473) and Total Akt in VSMCs pretreated with AS605240 (1 µM), followed by 10% FBS stimulation for 5 min (lower panel). G, Western blotting analysis of p-Akt (ser473) and Total Akt in PI3Kγ-KD VSMCs, followed by 10% FBS stimulation for 5 min (lower panel). (E, F and G) Bar graphs (upper panel) show the quantification of p-Akt level by the ratio of p-Akt to total Akt. Data are showed as mean ± SEM of six independent experiments. **P<0.01. H, Representative cross sections of ligated carotid arteries from WT and PI3Kγ–/– mice (7 and 21 days) immunostained for p-Akt (ser473) (left panel). IgG acted as the isotype control. Scale bars: 20 μm. Bar graph (right panel) shows the percentage of p-Akt-stained area to medial area. Data are showed as mean ± SEM. n=8 mice per group. * P<0.05, **P<0.01.
To further determine the expression of PI3Kγ in VSMCs, we conducted western blotting for class I PI3Ks in cultured VSMCs in vitro. We observed the expression of several PI3K isoforms, including p110α, p110β, p110γ, p110δ, and p101, in cultured VSMCs. Serum stimulation induced an increase in the protein levels of p110γ and p101, while there was no obvious change in the expression of p110α, p110β, or p110δ after serum treatment (Figure 1B). Moreover, immunofluorescent staining confirmed the upregulation of p110γ in the cytoplasm of VSMCs in response to serum (Figure 1C).
Following ligand binding, cell membrane receptors recruit and activate PI3Ks that phosphorylate PIP2 to generate PIP3, which in turn activates Akt and multiple downstream effectors to regulate cell proliferation and migration.12,13 We thus evaluated PI3Kγ activity in VSMCs by measuring phosphorylation of Akt (ser473) and PIP3 levels. Firstly, we constructed recombinant lentiviruses carrying Pik3cg-targeting shRNA and infected VSMCs. Our data showed that Pik3cg-targeting shRNA (PI3Kγ-KD) specifically inhibited p110γ expression but failed to affect regulator subunit p101 and other isoforms (p110α, p110β, p110δ) (Figure I in the online-only Data Supplement). Serum exposure caused a significant increase in PI3Kγ activity as measured by the production of PIP3 in VSMCs, as expected, PI3Kγ knockdown robustly suppressed the activity of PI3Kγ induced by serum (Figure 1D). Consistent with the effect on kinase activity, serum treatment led to enhanced phosphorylation of Akt (Figure 1E), while this effect was abolished by pretreatment with PI3Kγ inhibitor AS605240 (Figure 1F). Similarly, serum-induced Akt phosphorylation was dramatically attenuated by p110γ knockdown (Figure 1G). Furthermore, it was also confirmed in vivo by immunostaining of carotid arteries 7 and 21 days after ligation, demonstrating that vascular injury-induced Akt phosphorylation in medial VSMCs were significantly inhibited in PI3Kγ–/– arteries compared with WT arteries (Figure 1H). Taken together, these data suggest that vascular injury induces PI3Kγ upregulation and activates PI3Kγ signaling pathway in VSMCs.
PI3Kγ is required for VSMC phenotypic modulation following vascular injury.
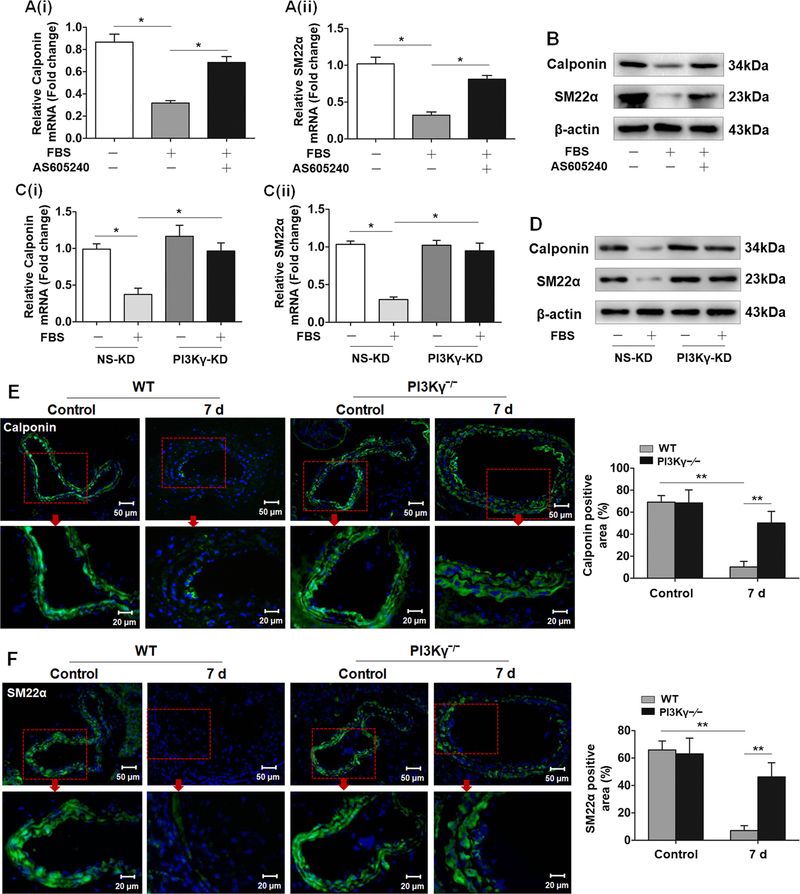
Several lines of evidence have uncovered specific roles for distinct PI3K isoforms in the cardiovascular system in both physiological and pathological process.27 However, the role of PI3Kγ in phenotypic modulation of VSMCs is still poorly elucidated. Thus, qRT-PCR and Western blotting were conducted to investigate the function of PI3Kγ in the expression of VSMC-specific marker genes Calponin and SM22α. The mRNA and protein levels of both Calponin and SM22α were decreased in serum-treated VSMCs, whereas the inhibition of Calponin and SM22α expression was largely diminished in VSMCs pretreated with AS605240 (Figure 2A and2B). Similarly, administration of serum also failed to diminish the expression of Calponin and SM22α in PI3Kγ-KD VSMCs (Figure 2C and2D). Additionally, the inhibition of regulatory subunit p101 has the same effect as p110 knockdown on Calponin and SM22α expression (Figure II in the online-only Data Supplement), supporting an important role of PI3Kγ signaling in regulating VSMC phenotype. To confirm these in vitro findings, we performed immunostaining for Calponin and SM22α in cross sections of carotid arteries. Consistent with the robust effect of serum on VSMC differentiation, vascular injury also strikingly suppressed the expression of Calponin and SM22α in the medial VSMCs of the ligated carotid arteries. In contrast, the injury-induced downregulation of Calponin and SM22α was almost completely abrogated in PI3Kγ–/– arteries (Figure 2E and2F). These results suggest that PI3Kγ contributes to induce VSMC phenotypic switching from a differentiated state to a dedifferentiated phenotype after vascular injury.
Figure 2. PI3Kγ is required for VSMC phenotypic modulation following vascular injury.
Cultured VSMCs pretreated with or without AS605240 (1 µM) were stimulated with 10% FBS. A, After 24 h, mRNA levels of Calponin (i) and SM22α (ii) were detected by qRT-PCR. mRNA expression was normalized to GAPDH. B, After 48 h, protein levels of Calponin and SM22α were determined by western blotting. C, qRT-PCR analysis of Calponin (i) and SM22α (ii) in PI3Kγ-KD VSMCs stimulated with 10% FBS for 24 h. D, Western blotting analysis of Calponin and SM22α in PI3Kγ-KD VSMCs stimulated with 10% FBS for 48 h. Data are showed as mean ± SEM of six independent experiments. * P<0.05. Representative cross sections of ligated carotid arteries from WT and PI3Kγ–/– mice (7 days) immunostained for Calponin (green) (left panel) (E) and SM22α (green) (left panel) (F). Cell nuclei were stained with hoechst (blue). Scale bar: 50 μm and 20 μm. Red boxes indicate the enlarged sections of carotid arteries. Corresponding bar graphs (right panel) show the percentage of Calponin and SM22α positive area in the media. Mean ± SEM. n=8 mice per group. **P<0.01.
PI3Kγ promotes VSMC proliferation and migration.
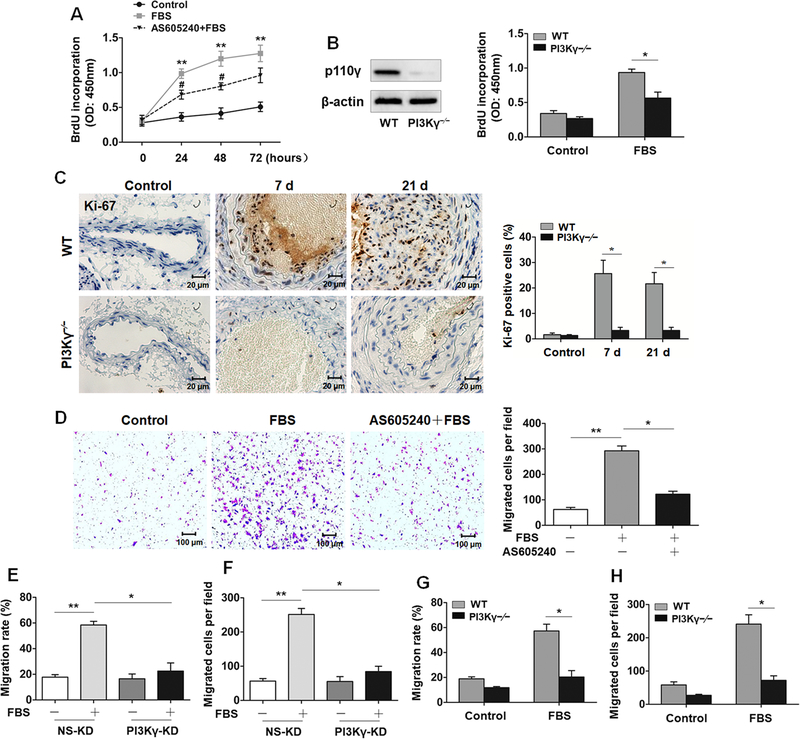
It is generally believed that phenotypic modulation may confer VSMCs with the capacities of promoting proliferation and migration into the neointimal layer, which contribute to vascular repair or even restenosis after vascular injury.1 PI3K/Akt signaling is involved in proliferation and migration in a variety of tissues.12 Therefore, we detected the effect of PI3Kγ signaling on serum-induced proliferation of VSMCs by pretreating VSMCs with AS605240, followed by serum stimulation utilizing time gradient method, and the proliferation of VSMCs measured by a BrdU proliferation assay and CCK-8 assay indicated that AS605240 attenuated the serum-induced proliferation of VSMCs (Figure 3A, Figure IIIA in the online-only Data Supplement). Furthermore, the proliferation ability conferred by serum was also attenuated in PI3Kγ–/– VSMCs (Figure 3B). In vivo, an increase in Ki-67-positive cells within the medial VSMCs of ligated carotid arteries were significantly reduced by PI3Kγ deletion, demonstrating a crucial involvement of PI3Kγ signaling in proliferative response of dedifferentiated VSMCs (Figure 3C). Consistent with the effect of serum on cell proliferation, serum-treated VSMCs exhibited accelerated migration ability measured by a wound-healing assay and Transwell assay. As predicted, VSMCs pretreated with AS605240 followed by serum incubation migrated slower than the serum-treated group (Figure IIIB in the online-only Data Supplement). AS605240 pretreatment also diminished the amount of VSMCs moving through chamber polycarbonate membrane (Figure 3D). Subsequently, the robust inhibition of VSMCs migration was further verified in PI3Kγ-KD VSMCs (Figure 3E and3F) and PI3Kγ–/– VSMCs (Figure 3G and3H). Taken together, PI3Kγ exerts a positive role in not only the proliferative but the migratory process.
Figure 3. PI3Kγ promotes VSMC proliferation and migration.
A, VSMCs pretreated with or without AS605240 (1 µM) were stimulated with 10% FBS for indicated time, cells growth were evaluated by BrdU incorporation assay. B, Western blotting analysis of p110γ expression in vessel extracted from WT and PI3Kγ–/– mice (left panel). Proliferation of PI3Kγ–/– VSMCs in response to 10% FBS stimulation for 48 h was evaluated by BrdU incorporation assay (right panel). C, Representative cross sections of carotid arteries from WT and PI3Kγ–/– mice (7 and 21 days) immunostained for Ki-67 (left panel). Scale bars: 20 μm. Bar graph (right panel) shows the percentage of Ki-67-stained positive cells in vascular media. Data are showed as mean ± SEM. n=8 mice per group. D, VSMCs pretreated with AS605240 were stimulated with 10% FBS for 48 h, representative images of migrated VSMCs on the bottom of transwell membrane (left panel). Scale bar: 100 μm. Bar graph (right panel) shows the number of migrated cells. The migration ability of PI3Kγ-KD VSMCs was evaluated by wound-healing assay (E) and Transwell assay (F). The migration ability of PI3Kγ–/– VSMCs was evaluated by wound-healing assay (G) and Transwell assay (H). Data are showed as mean ± SEM of at least 4 independent experiments. * P<0.05, **P<0.01.
Induction of YAP mediates the effects of PI3Kγ on VSMC phenotypic modulation.
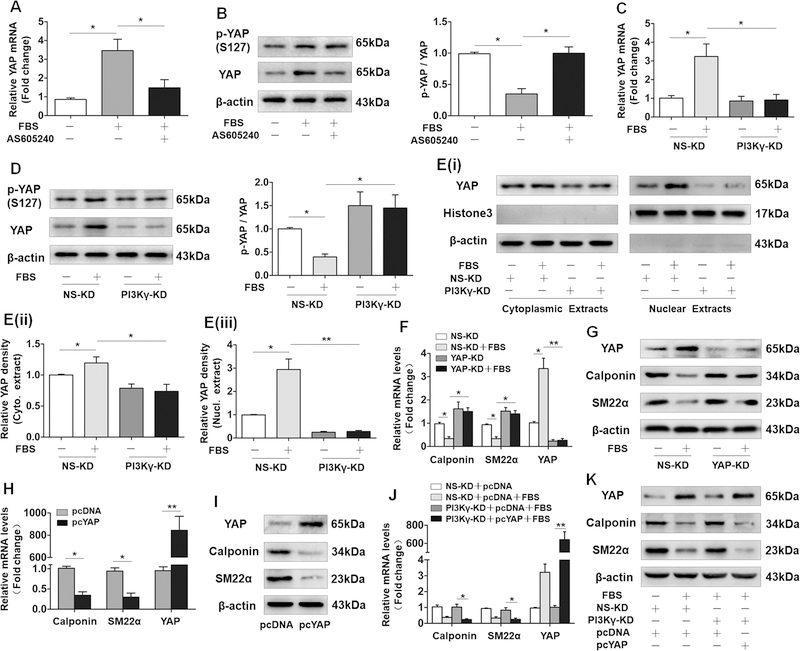
YAP is known to be crucial for VSMC phenotypic modulation by repressing the expression of VSMC marker genes.10,11 Given the identification of PI3K pathway in YAP expression and YAP phosphorylation at serine 127 (S127), 28,29 we hypothesized that PI3Kγ signaling contributes to VSMC phenotypic modulation through mediating YAP. As is shown in Figure 4A, serum treatment triggered a marked increase in mRNA level of YAP, whereas the upregulation of YAP was largely inhibited by AS605240 pretreatment. In protein level, along with the increased mRNA expression, total YAP and p-YAP, especially total YAP were drastically increased by serum, AS605240 pretreatment also significantly blocked serum-induced upregulation of total YAP, while failed to effectively inhibit the phosphorylation of YAP, conversely, the proportion of p-YAP to total YAP was elevated (Figure 4B). Similarly, this phenomenon was observed in PI3Kγ-KD VSMCs exposed to serum (Figure 4C and4D). Previous observations that phosphorylation of YAP at S127 results in cytoplasmic localization. 30 Of relevance, PI3Kγ knockdown significantly attenuated the serum-induced nuclear accumulation of YAP in VSMCs (Figure 4E). We next measured YAP expression in vivo, immunostaining for YAP in ligated carotid arteries demonstrated that PI3Kγ deficiency strikingly reduced vascular injury-induced upregulation of YAP (Figure IV in the online-only Data Supplement). Collectively, these data indicated a crucial role of PI3Kγ in controlling YAP expression and nuclear localization in VSMCs. Moreover, consistent with previous studies,10,11 YAP knockdown using YAP-targeting shRNA drastically abrogated serum-induced downregulation of Calponin and SM22α (Figure 4F and4G). In contrast, ectopic expression of YAP induced by transfecting VSMCs with plasmids carrying YAP gene (pcYAP) led to a drastic suppression of VSMC marker genes (Figure 4H and4I). Subsequently, to confirm the role of YAP in controlling VSMC differentiation in the downstream of PI3Kγ signaling, we performed combined transfection of PI3Kγ shRNA and pcYAP and corresponding control in VSMCs prior to serum stimulation. As expected, ectopic expression of YAP significantly rescued the serum-induced suppression of VSMC differentiation in PI3Kγ-KD VSMCs (Figure 4J and4K). Collectively, these data suggest that induction of YAP promotes VSMC phenotypic modulation through PI3Kγ signaling.
Figure 4. Induction of YAP mediates the effects of PI3Kγ on VSMC phenotypic modulation.
VSMCs pretreated with or without AS605240 were stimulated with 10% FBS, (A) mRNA level of YAP was determined after 24 h, (B) protein levels of p-YAP and YAP was measured after 48 h (left panel), bar graph (right panel) shows the relative ratio of p-YAP to YAP. (C) YAP mRNA and (D) YAP protein expression, p-YAP level (left panel) in PI3Kγ-KD VSMCs stimulated with 10% FBS, bar graph (right panel) shows the relative ratio of p-YAP to YAP. E, Cytoplasmic and nuclear extracts from PI3Kγ-KD VSMCs treated with FBS were immunoblotted using anti-YAP (i). Bar graphs show densitometric analysis of YAP expression in cytoplasmic extracts (ii) and nuclear extracts (iii). VSMCs were transfected with YAP shRNA (YAP-KD) and its corresponding negative control (NS-KD) followed by 10% FBS stimulation, mRNA levels of Calponin, SM22α and YAP were detected after 24 h (F), and protein levels were determined after 48 h (G). qRT-PCR (H) and western blotting (I) analyses of YAP, Calponin and SM22α in VSMCs transfected with plasmid overexpressing YAP (pcYAP) and corresponding control (pcDNA). VSMCs were co-transfected with PI3Kγ shRNA and pcYAP plasmid prior to 10% FBS stimulation, mRNA levels of Calponin, SM22α and YAP were detected (J), protein levels were determined (K). All data are presented as mean ± SEM of six independent experiments. * P<0.05, **P<0.01.
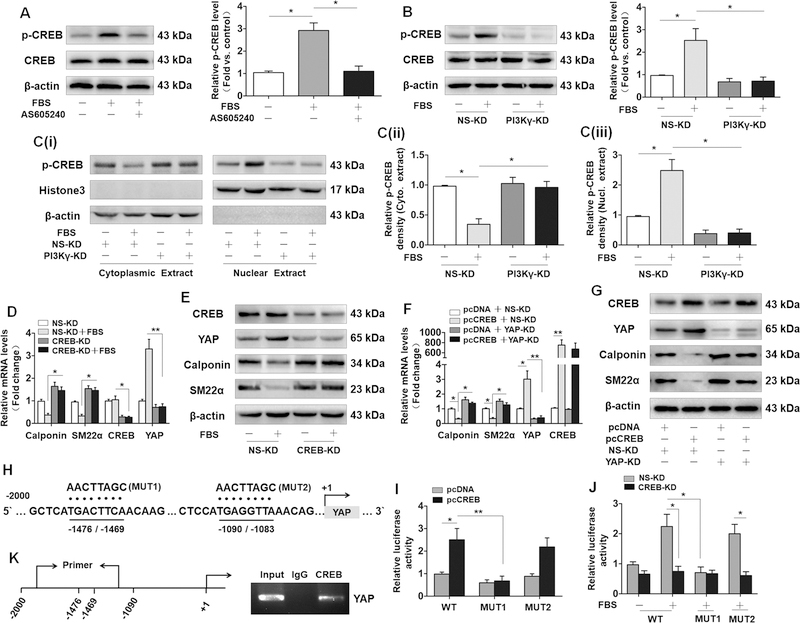
CREB activation is responsible for PI3Kγ-induced YAP expression and VSMC phenotypic modulation.
It has been reported that nuclear factor CREB contributes to the expression of YAP by binding its promoter region in human hepatoma cells.31 Moreover, Akt/PKB has been identified as an important regulator inducing the phosphorylation of CREB at Ser-133.29 Based on the previous studies, we speculated that PI3Kγ mediates YAP expression in a CREB-dependent manner and CREB activity may play a pivotal role in phenotypic modulation of VSMCs induced by serum. To test this hypothesis, we firstly measured the effect of serum on the CREB activity in cultured VSMCs, and our results indicated that serum treatment drastically increased the phosphorylation of CREB, while the effect was strikingly abrogated by using AS605240 (Figure 5A) and transfection of PI3Kγ shRNA (Figure 5B). Intriguingly, we observed that administration of serum resulted in increased nuclear localization of phosphorylated CREB, which could also be blocked by PI3Kγ knockdown as measured by western blotting (Figure 5C). We next performed immunostaining for p-CREB to examined p-CREB expression in vivo. Consistent with these in vitro data, injury-induced increase in p-CREB within medial VSMCs of carotid arteries was also attenuated in PI3Kγ–/– arteries (Figure V in the online-only Data Supplement), indicating a crucial role of PI3Kγ signaling in the activation of CREB. Subsequently, we explored the effect of CREB on YAP expression and VSMC phenotypic modulation. As predicted, CREB knockdown strikingly abolished serum-induced YAP upregulation but drastically reversed the downregulation of VSMCs marker genes induced by serum (Figure 5D and5E). Conversely, the ectopic expression of CREB conferred by transfecting VSMCs with plasmids carrying CREB gene (pcCREB) in VSMCs induced a marked upregulation of YAP but resulted in a drastic reduction of VSMC marker genes, while the effects of CREB overexpression on YAP, Calponin and SM22α were markedly diminished in YAP-KD VSMCs (Figure 5F and5G), suggesting that CREB activation contributes to phenotypic modulation of VSMCs through inducing YAP upregulation.
Figure 5. CREB activation is responsible for PI3Kγ-induced YAP expression and VSMC phenotypic modulation.
A, Western blotting analysis of p-CREB and CREB in VSMCs pretreated with AS605240 (1 µM), followed by 10% FBS treatment for 5 min (left panel). B, Western blotting analysis of p-CREB and CREB in PI3Kγ-KD VSMCs stimulated with 10% FBS for 5 min (left panel). (A and B) Corresponding bar graph (right panel) shows the quantification of p-CREB level by the ratio of p-CREB to total CREB. C, Western blotting analysis of p-CREB in cytoplasmic extracts and nuclear extracts from PI3Kγ-KD VSMCs stimulated with 10% FBS for 5 min (left panel). Bar graph shows the densitometric analysis of p-CREB in cytoplasmic (middle panel) and nuclear extracts (right panel). D, VSMCs were transfected with CREB shRNA (CREB-KD) followed by 10% FBS stimulation for 24h, the mRNA level of YAP, CREB, Calponin and SM22α was detected. E, Western blotting analysis of these genes in CREB-KD VSMCs stimulated with 10% FBS for 48 h. qRT-PCR (F) and western blotting (G) analyses of YAP, CREB, Calponin and SM22α in VSMCs cotransfected with CREB plasmid (pcCREB) and YAP shRNA (YAP-KD). H, Two putative CREB binding motifs (−1476 / −1469 and −1090 / −1083) within the upstream of the transcription start site of YAP are underlined (WT). The mutated sequences (MUT1 and MUT2) are denoted by dots. I, CREB expression plasmid (pcCREB) and its corresponding control (pcDNA) were cotransfected with WT pGL3-YAP-promoter plasmid or mutant reporter plasmid (MUT1 and MUT2) into VSMCs. Luciferase reporter gene assay was performed 48 hours after transfection. J, CREB shRNA was cotransfected with WT pGL3-YAP-promoter plasmid or mutant reporter plasmid (MUT1 and MUT2) into VSMCs, followed by 10% FBS treatment for 24 h. Promoter activity of YAP was measured by luciferase reporter gene assay. K, The binding of CREB to the promoter region of YAP (−1476 / −1469) was verified by CHIP assays (right panel), primer covering the binding region (−1476 / −1469) was designed (left panel). Data are presented as mean ± SEM of six independent experiments. * P<0.05, **P<0.01.
To determine whether CREB upregulates YAP expression in VSMCs at the transcription level, we inspected the promoter region of YAP and identified two putative CREB binding motifs (−1476 / −1469 and −1090 / −1083) within the upstream of the transcription start site of YAP (Figure 5H). Then, mutants (MUT 1 and MUT 2) were constructed by site-directed mutation within potential binding sequences. Luciferase reporter plasmids containing putative binding sequences (wild-type, WT) or mutated sequence were cotransfected with CREB expression plasmid (pcCREB) or CREB shRNA (CREB-KD) into VSMCs. The luciferase reporter gene assays demonstrated a decrease inYAP promoter activity from MUT1 compared with the wild-type (WT). Ectopic expression of CREB robustly enhanced YAP promoter activity from WT and MUT 2 but failed to affect the promoter activity from MUT1 (Figure 5I). Accordingly, serum exposure markedly induced the promoter activity of YAP from WT and MUT 2, whereas the increased promoter activity was robustly abolished by CREB knockdown or mutation of bind site (−1476 / −1469) from MUT1 but not by MUT2 (Figure 5J). Then, CHIP assay was performed to further identify the physical binding of endogenous CREB to the putative sequence (−1476 / −1469) in YAP promoter using anti-CREB (Figure 5K). Taken together, these data indicated that −1476 / −1469 within YAP promoter may be functional bind motif for CREB that promotes YAP transcription expression.
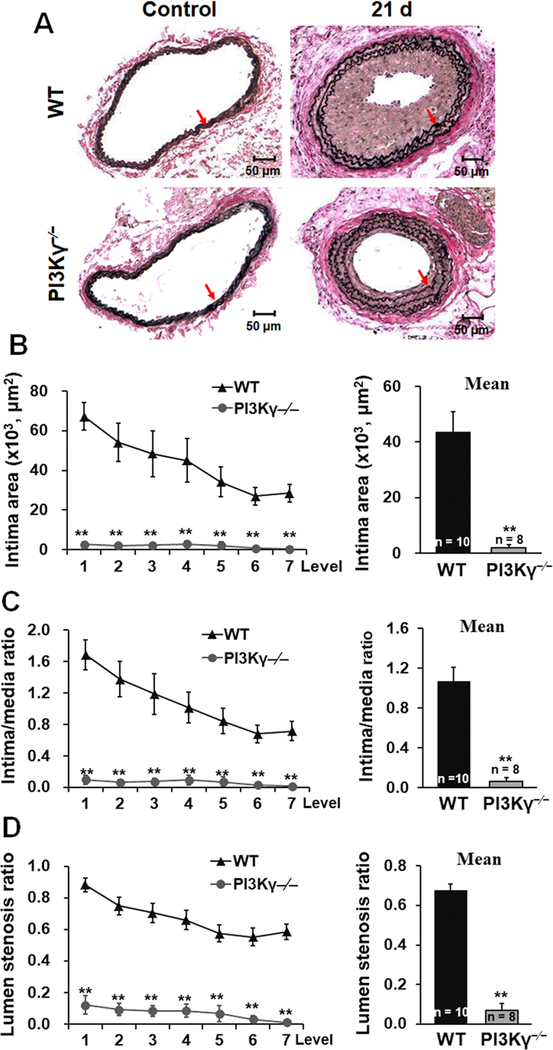
PI3Kγ deficiency attenuates neointimal formation following arterial injury.
Vascular injury induces phenotypic modulation of medial VSMC of injured artery followed by neointimal formation and vascular remodeling. To determine the functional role of PI3Kγ in vascular lesion formation and remodeling, we used an established model of carotid artery ligation in mice. No neointimal formation and luminal narrowing were observed in the unligated right common carotid arteries of WT and PI3Kγ–/– mice. In contrast, carotid ligation led to the development of a prominent neointima in left common carotid arteries of WT mice, but only a small lesion in PI3Kγ–/– mice 21 days after carotid artery ligation (Figure 6A). Compared with WT mice, neointimal formation as determined by intima area (Figure 6B) and intima/media ratio (Figure 6C) in injured arteries were significantly attenuated in PI3Kγ–/– mice, resulting in a marked decrease of lumen stenosis ratio (Figure 6D). These findings indicate that PI3Kγ plays a crucial role of in the development of neointimal formation after carotid artery ligation.
Figure 6. PI3Kγ deficiency attenuates neointimal formation following arterial injury.
A, Representative Verhoeff’s Elastic stained cross sections (level 3) of carotid arteries from WT and PI3Kγ−/− mice at 21d after ligation. Red arrows indicate the internal elastic lamina. Scale bars: 50 μm. B, Intima area was determined at 7 cross section level (120 μm intervals), and their mean value was calculated. Intima/media ratio (C) and lumen stenosis ratio (D) at each level and the mean ratios were calculated. Numbers at the base of the bar graphs indicate the number of mice in each group. Mean ± SEM. **P<0.01 versus corresponding WT group.
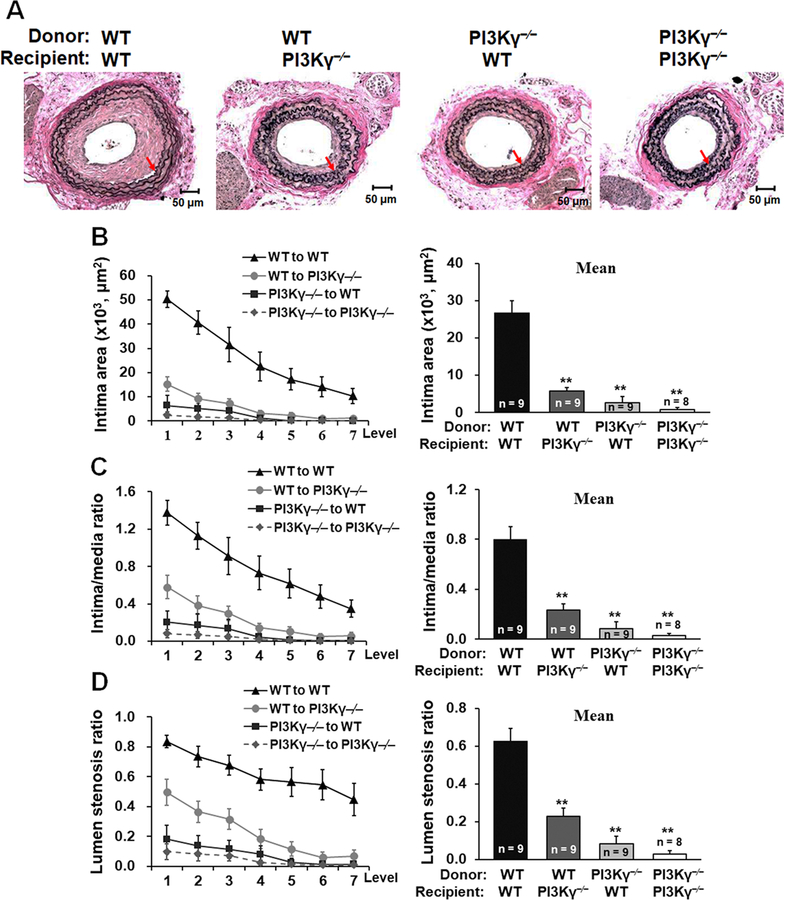
PI3Kγ in local vascular cells and in BM-derived cells both contribute to neointimal formation.
PI3Kγ is expressed not only in local vascular cells but also in bone marrow-derived cells, both of which are active players in neointimal formation in response to vascular injury. However, the relative contributions of local vascular cell-derived PI3Kγ versus BM cell-derived PI3Kγ to the development of neointimal lesions are unknown. Therefore, we performed reciprocal BM transplantation from PI3Kγ–/– or WT donors into PI3Kγ–/– or WT recipient mice to create BM chimeric mice. Mice underwent carotid artery ligation injury, and neointimal formation was measured 21 days later. Interestingly, neointimal formation was greatly smaller either in WT to PI3Kγ–/– mice or in PI3Kγ–/– to WT mice compared with that of WT to WT mice. In addition, PI3Kγ–/– to PI3Kγ–/– mice exhibited less neointimal formation and diminished lumen stenosis ratio compared with those of WT to PI3Kγ–/– mice or PI3Kγ–/– to WT mice (Figure 7, A-D). Taken together, our data suggest that PI3Kγ in local vascular cells and in BM-derived cells both critically contribute to neointimal formation following vascular injury.
Figure 7. PI3Kγ in local vascular cells and in BM-derived cells both contribute to neointimal formation.
A, Representative Verhoeff’s Elastic stained cross sections (level 3) of carotid arteries from chimeric mice at 21d after ligation. Red arrows indicate the internal elastic lamina. Scale bars: 50 μm. B, Intima area was determined at 7 cross section level (120 μm intervals), and their mean value was calculated. Intima/media ratio (C) and lumen stenosis ratio (D) at each level and the mean ratios were calculated. Numbers at the base of the bar graphs indicate the number of mice in each group. **P<0.01 versus corresponding WT to WT group.
Discussion
Vascular injury such as balloon angioplasty and vascular stenting triggers the early release of proinflammatory cytokines and growth factors that induce medial VSMC activation and phenotypic modulation toward the synthetic state, leading to enhanced migration and proliferation, which contribute to the development of neointimal lesions in the injured artery.1,2 Therefore, VSMCs are the active players in the pathogenesis of neointimal formation and restenosis after vascular injury. PI3Kγ is the only member of class IB PI3Ks activated by G protein-coupled receptors via binding to βγ subunits of G proteins. PI3Kγ has emerged as a key signaling molecule in immune and proinflammatory signaling pathways activated by many different stimuli.13,32 Of relevance, PI3Kγ have important functions in immune cells and endothelial cells, and the activities of these cells are crucial in the pathogenesis of atherosclerosis and restenosis after vascular injury.33,34 Also, PI3Kγ has been found in VSMCs.16,17 However, the function of PI3Kγ in regulating VSMC behaviors and the underlying mechanisms remain to be clarified. Here, we present the novel findings that PI3Kγ plays a critical role in phenotypic modulation of VSMCs in response to vascular injury in vivo or serum stimulation in vitro. PI3Kγ-mediated CREB activation increases YAP expression, leading to VSMC phenotypic modulation. Blockade of PI3Kγ signaling abolishes the effect of serum on VSMCs and strikingly attenuates neointimal formation in injured artery. These results reveal previously uncharacterized function of PI3Kγ in mediating VSMC phenotypic modulation and its involvement in neointimal formation in response to vascular injury.
PI3Ks are lipid signaling enzymes that participates in a variety of cellular processes and whose activity is directly proportional to the expression level of PI3K subunits.35,36 Previously, PI3K isoforms such as p110α, p110δ and p110γ have been found to be overexpressed in several different cancers.36,37 Of relevance, overexpression of PI3K isoforms is sufficient to induce cellular transformation and proliferation, which are attributed to enhanced PI3K activity and increased level of Akt phosphorylation.36,37 PI3Kγ upregulation is observed in human and murine atherosclerotic lesions and intimately linked to the development of atherosclerosis.33,38 In addition, myocardial infarction or tissue ischemia can induce the expression of PI3Kγ and that contributes to reparative neovascularization and wound healing through modulating endothelial proliferation, survival, and migration.39,40 In idiopathic pulmonary fibrosis lung tissue and fibroblasts, enhanced expression of PI3Kγ is consistent with increased Akt activation and cellular proliferation.41 For vascular injury, the proliferation and migration of medial VSMCs are the main sources of neointimal formation.1 Some inflammatory/growth factors (TNF-α, IL-1β and PDGF) in local tissues/ plasma and lysophosphatidic acid (LPA) in serum are believed to possess potent mitogenic effects that contributes to VSMC activation and dedifferentiation.1,42 Although the composition of serum is complex, current studies have identified that TNF-α and LPA can bind to G protein-coupled receptors, leading to p110γ activation.43,44 Accordingly, we detected robust PI3Kγ upregulation in the medial and intimal region of the injured arteries as well as in cultured VSMCs upon serum exposure, accompanying with increased cellular proliferation and decreased expression of VSMC differentiation markers. More importantly, the present study reveals a positive correlation between PI3Kγ expression and VSMC phenotypic modulation in response to vascular injury, implying that PI3Kγ-induced VSMC phenotypic modulation constitutes a mechanism accounting for accelerated neointimal formation and vascular remodeling after vascular injury.
PI3Kγ is primarily expressed in hematopoietic cells and implicated in the regulation of inflammation, which is critical for the development of neointimal formation and vascular remodeling.15,34 Indeed, PI3Kγ has previously been shown to critically contribute to neointimal hyperplasia through modulating inflammatory response to environmental stimuli. In atherosclerosis, recent studies in animal models have identified PI3Kγ as an essential player in vascular inflammatory processes, as its pharmacological inhibition or its genetic deletion leads to reduced monocyte and T cell recruitment and activation in arterial lesions, resulting in impaired atherosclerotic plaque development and increased plaque stabilization.33,38 Previous work from our group also demonstrated that platelet PI3Kγ plays a major role in controlling vascular inflammation and intima-media thickening by modulating platelet activation following vascular injury.45 Furthermore, PI3Kγ in bone marrow-derived cells has also been shown to be crucial for the development of neointimal formation through specifically modulating Th1 cytokine profile that is responsible for inducing VSMC proinflammatory phenotype and leukocyte infiltration in injured arteries.34 Consistently, our results from bone marrow transplantation experiments also confirm the important role of bone marrow-derived PI3Kγ in mediating neointimal formation and vascular remodeling after vascular injury. Additionally, the present study provides novel evidence that vascular wall cell-derived PI3Kγ is responsible, at least in part, for injury-induced VSMC phenotypic modulation that contributes to neointima development. Therefore, these findings suggest a potential involvement of local vascular PI3Kγ in the pathogenesis of vascular proliferative diseases.
Like class IA PI3K isoforms, PI3Kγ functions as an important signal transduction enzyme that regulate diverse biological functions of multiple cell types by acting downstream of cell surface receptor activation. It has been shown that PI3Kγ is involved in controlling numerous functions of immune cells such as thymocyte development,19 T cell activation,34 neutrophil trafficking and the oxidative burst,19 NK cell development and cytotoxicity,46 and monocyte/macrophage activation,32,38 and these functions of PI3Kγ in regulating immune cells actively contribute to the development of chronic inflammation, atherosclerosis, and cancer. In cardiomyocytes, PI3Kα modulates cell growth and apoptosis, while PI3Kγ contributes to TAC-induced cardiac remodeling in a kinase-dependent activity and negatively regulates cardiac contractility by a kinase-independent effect.47,48 Recently, PI3Kγ has also been shown to modulate cell proliferation, survival, and migration of vascular endothelial cells that contribute to angiogenesis and vasculogenesis.40 In addition, emerging evidence indicates that PI3Kγ is essential for both MCP-1-induced VSMC migration17 and AngII-evoked VSMC contractility.16 This is further evident by our findings that pharmacological and genetic inhibition of PI3Kγ abrogates increased cell proliferation and migration and reduced expression of VSMC-specific contractile genes in VSMCs induced by serum, suggesting the critical role of PI3Kγ in controlling VSMC phenotypic modulation. In support of our findings, PI3K signaling has been shown to play an important role in mediating VSMC phenotypic switching in response to diverse extracellular stimuli. Upon stimulation by PDGF or serum, activation of PI3K/Akt signaling leads to VSMC phenotypic switching into the synthetic phenotype associated with enhanced cellular proliferation and migration. In contrast, disruption of PI3K/Akt pathway inhibits VSMC proliferation and neointimal hyperplasia after vascular injury.49–51 In agreement with these previous studies, we found that genetic deletion of PI3Kγ results in reduced cellular proliferation and impaired neointimal lesion formation in injured carotid artery, suggesting a key role of PI3Kγ in the regulation of VSMC activation and neointimal formation after vascular injury. However, it should be noted that the highly homologous isoforms of AKT, Akt1 and Akt2, exert different functions in VSMC.52 Current evidences have identified the crucial function of Akt1 in promoting VSMC proliferation and migration.53,54 As opposed to Akt1, Akt2 knockdown in VSMCs attenuated Myocardin expression that results in a more dedifferentiated phenotype, accordingly, genetic deletion of Akt2 promotes VSMC proliferation and intimal hyperplasia after artery injury.55 Given the findings in present study, the mechanism of PI3Kγ mediates VSMC phenotypic modulation via Akt1 may be established, and further research should be performed.
Strikingly, this study identified YAP as a downstream effector of PI3Kγ in VSMC phenotypic modulation. YAP is a nuclear effector of Hippo signaling that controls cellular survival and proliferation via binding to DNA-binding transcriptional factor to induce gene expression.7 YAP has been proposed to be implicated in VSMC phenotypic modulation and neointimal formation.10,11 Our results are in accordance with previous studies showing that the expression level of YAP is correlated with the VSMC synthetic phenotype. More importantly, the present study also indicates a direct correlation between PI3Kγ activity and YAP expression as well as VSMC phenotypic modulation, implying that YAP expression constitutes a molecular mechanism accounting for PI3Kγ-induced VSMC phenotypic modulation. Besides its expression, YAP activation is regulated by phosphorylation-dependent nuclear translocation, which depends on the Lats kinase, a key component of the Hippo pathway.8 Consistent with previous studies, 28 our evidence shown that the activation of PI3Kγ signaling induced by serum is responsible for YAP dephosphorylation and nuclear accumulation. Accordingly, nuclear YAP protein interacts with myocardin and suppresses the binding of myocardin to SRF in VSMCs, leading to attenuated expression of VSMC contractile markers.10,11 These data suggest that aberrant YAP expression and activation may represent an important mechanism underlying PI3Kγ-induced VSMC phenotypic modulation.
Another important finding of this study is the identification of CREB as a key transcriptional regulator in the downstream of PI3Kγ signaling for promoting YAP expression and VSMC phenotypic modulation. In support of these findings, it has been shown that CREB plays a key role in modulating the transcription of YAP in several cell types.29,31,56 Notably, a variety of protein kinase pathways, including PI3K/Akt pathway, have been shown to modulate CREB function through phosphorylation of CREB at serine 133. 29,56,57 This concept is also confirmed by our observations, indicating that disruption of PI3Kγ signaling is sufficient to abolish serum-induced CREB phosphorylation and transcriptional activity. CREB is a nuclear transcription factor that binds to CRE sequences on promoters of target genes, thereby activating transcription of genes important to cellular survival, growth, migration, and differentiation in multiple cell types.58 CREB has been shown to be critically involved in the regulation of VSMC functions as well as vascular remodeling. Several studies indicated that vascular injury induces CREB phosphorylation at Ser133 in VSMCs and that is positively associated with neointimal formation in injured artery. Blocking CREB activation with overexpression of either dominant-negative CREB or A20 leads to attenuated neointimal formation after arterial injury.57,59–61 In response to mitogenic stimuli such as TNF-α, PDGF, angiotensin II, and thrombin, VSMCs display elevated level of CREB phosphorylation thereby promoting cellular migration and proliferation in vitro.58,62,63 This is further evident by our findings that serum induces CREB phosphorylation and nuclear translocation, leading to enhanced expression of YAP, causing a reduced expression of VSMC contractile genes and consequently VSMC phenotypic modulation. However, several studies have reported a negative correlation between CREB content and VSMC proliferation as well as migration.64,65 This discrepancy may be attributable to differences in cell resources and experimental design. Despite some contradictory observations, multiple lines of evidence point to the importance of CREB in controlling YAP expression and VSMC activation as well as neointimal formation after vascular injury.
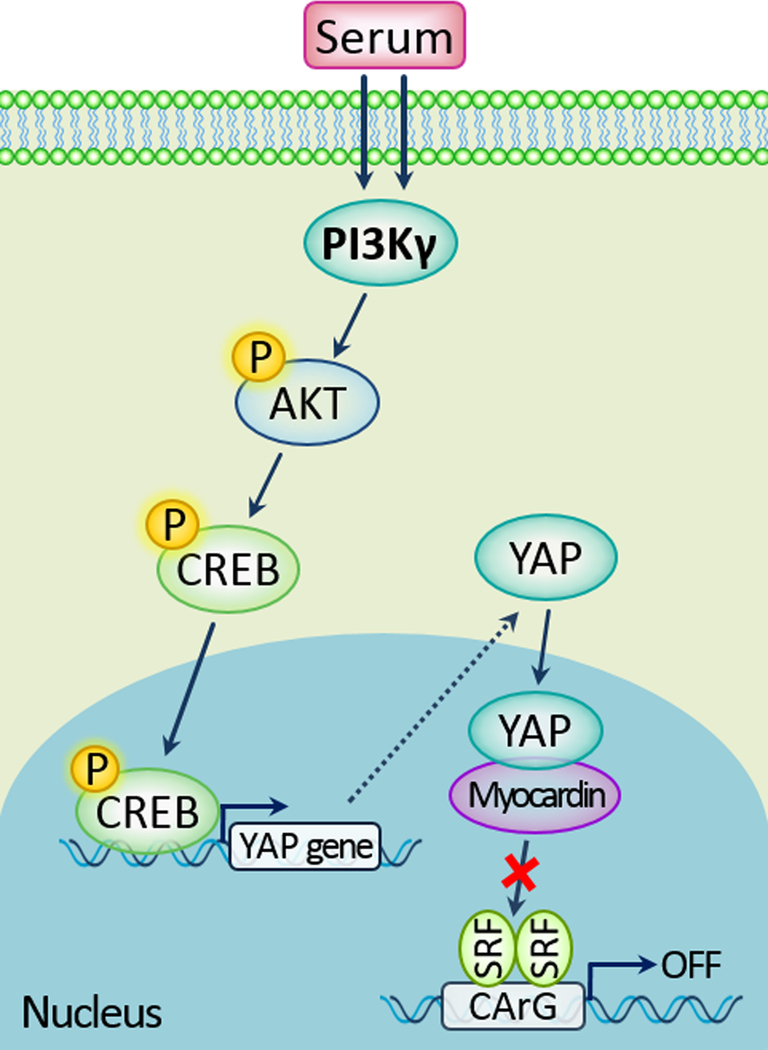
In summary, our present study identified PI3Kγ as a pivotal regulator in promoting VSMC phenotypic modulation through modulating, at least in part, the CREB/YAP signaling pathway (Figure 8). Our findings support the notion that PI3Kγ in VSMCs plays a critical role in the development of neointimal formation in response to vascular injury. Therefore, local vascular cell-derived PI3Kγ may represent an attractive therapeutic target for the treatment of vascular proliferative diseases.
Figure 8.
A model shows the signaling crosstalk between FBS, PI3Kγ activity, p-CREB, YAP and VSMC phenotypic modulation.
Supplementary Material
Highlights.
Vascular injury induces PI3Kγ upregulation and activates PI3Kγ/Akt signaling pathway in VSMCs.
PI3Kγ transcriptional activation contributes to VSMC phenotypic modulation, leading to increased cellular proliferation and migration.
PI3Kγ controls VSMC phenotypic modulation through regulating transcription factor CREB activation and YAP expression.
Vascular wall cell-derived PI3Kγ deficiency represses neointimal formation after vascular injury.
Acknowledgments
We thank Josef M. Penninger (Institute for Molecular Biotechnology of the Austrian Academy of Sciences, Vienna, Austria) for kindly providing PI3K-p110γ knockout (PI3Kγ–/–) mice (C57BL/6J background).
Sources of Funding
This study was supported by the research grants from the National Natural Science Foundation of China to Z.S. (No. 81370582, 81170441, 81670374) and by the Louisiana State University Health Sciences Foundation Fund for Schumpert Endowed Chair (G.L.) and the National Institutes of Health grant NS089991 (G.L.)
Nonstandard Abbreviations and Acronyms
- VSMCs
vascular smooth muscle cells
- PI3Kγ
phosphatidylinositol 3-kinase γ
- YAP
yes-associated protein
- CREB
cyclic AMP-response element binding protein
- p-CREB
phosphorylated cyclic AMP-response element binding protein
- SRF
serum response factor
- MCP-1
monocyte chemoattractant protein-1
- PDGF
platelet derived growth factor
- TNF-α
tumor necrosis factor-α
- WT
wild type
- Mst1/2
mammalian sterile 20-like 1/2
- Lats1/2
large tumor suppressor homolog 1/2
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Disclosure
None
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 2.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 2012;95:194–204. [DOI] [PubMed] [Google Scholar]
- 3.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012;74:13–40. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 2016;118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 2006;116:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 2004;428:185–189. [DOI] [PubMed] [Google Scholar]
- 7.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015;163:811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol 2015;25:499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008;22:1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Hu G, Gao X, Wang Y, Zhang W, Harmon EY, Zhi X, Xu Z, Lennartz MR, Barroso M, Trebak M, Chen C, Zhou J. The induction of yes-associated protein expression after arterial injury is crucial for smooth muscle phenotypic modulation and neointima formation. Arterioscler Thromb Vasc Biol 2012;32:2662–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie C, Guo Y, Zhu T, Zhang J, Ma PX, Chen YE. Yap1 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin. J Biol Chem 2012;287:14598–14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans 2006;34:647–662. [DOI] [PubMed] [Google Scholar]
- 13.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 2010;11:329–341. [DOI] [PubMed] [Google Scholar]
- 14.Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, Hawkins PT, Stephens L. Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol 2006;8:1303–1309. [DOI] [PubMed] [Google Scholar]
- 15.Barberis L, Hirsch E. Targeting phosphoinositide 3-kinase gamma to fight inflammation and more. Thromb Haemost 2008;99:279–285. [DOI] [PubMed] [Google Scholar]
- 16.Vecchione C, Patrucco E, Marino G, et al. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J Exp Med 2005;201:1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fougerat A, Smirnova NF, Gayral S, Malet N, Hirsch E, Wymann MP, Perret B, Martinez LO, Douillon M, Laffargue M. Key role of PI3Kgamma in monocyte chemotactic protein-1-mediated amplification of PDGF-induced aortic smooth muscle cell migration. Br J Pharmacol 2012;166:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrotta M, Lembo G, Carnevale D. The Multifaceted Roles of PI3Kgamma in Hypertension, Vascular Biology, and Inflammation. Int J Mol Sci 2016;17:E1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 2000;287:1040–1046. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Oparil S, Chen YF, McCrory MA, Skibinski GA, Feng W, Szalai AJ. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol 2005;25:2094–2099. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y, Xu J. Loss-of-function deletion of the steroid receptor coactivator-1 gene in mice reduces estrogen effect on the vascular injury response. Arterioscler Thromb Vasc Biol 2007;27:1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Liu S, Li W, Hu S, Xiong J, Shu X, Hu Q, Zheng Q, Song Z. Vascular smooth muscle cell apoptosis promotes transplant arteriosclerosis through inducing the production of SDF-1alpha. Am J Transplant 2012;12:2029–2043. [DOI] [PubMed] [Google Scholar]
- 23.Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 2005;11:936–943. [DOI] [PubMed] [Google Scholar]
- 24.Yang B, Li W, Zheng Q, et al. Transforming growth factor beta-activated kinase 1 negatively regulates interleukin-1alpha-induced stromal-derived factor-1 expression in vascular smooth muscle cells. Biochem Biophys Res Commun 2015;463:130–136. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Li W, Yu Q, Guo B, Yang B, Zhang C, Li M, Li J, Hu S, Zheng Q, Song Z. High Mobility Group Box 1 Mediates Interferon-gamma-Induced Phenotypic Modulation of Vascular Smooth Muscle Cells. J Cell Biochem 2017;118:518–529. [DOI] [PubMed] [Google Scholar]
- 26.Song Z, Jin R, Yu S, Nanda A, Granger DN, Li G. Crucial role of CD40 signaling in vascular wall cells in neointimal formation and vascular remodeling after vascular interventions. Arterioscler Thromb Vasc Biol 2012;32(1):50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res 2009;82:261–271. [DOI] [PubMed] [Google Scholar]
- 28.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A 2013;110:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Harris RC. Interaction of the EGF Receptor and the Hippo Pathway in the Diabetic Kidney. J Am Soc Nephrol 2016;27:1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007;21:2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Zhang J, You X, Liu Q, Du Y, Gao Y, Shan C, Kong G, Wang Y, Yang X, Ye L, Zhang X. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology 2012;56:2051–2059. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000;287:1049–1053. [DOI] [PubMed] [Google Scholar]
- 33.Fougerat A, Gayral S, Gourdy P, Schambourg A, Ruckle T, Schwarz MK, Rommel C, Hirsch E, Arnal JF, Salles JP, Perret B, Breton-Douillon M, Wymann MP, Laffargue M. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation 2008;117:1310–1317. [DOI] [PubMed] [Google Scholar]
- 34.Smirnova NF, Gayral S, Pedros C, et al. Targeting PI3Kgamma activity decreases vascular trauma-induced intimal hyperplasia through modulation of the Th1 response. J Exp Med 2014;211:1779–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghigo A, Laffargue M, Li M, Hirsch E. PI3K and Calcium Signaling in Cardiovascular Disease. Circ Res 2017;121:282–292. [DOI] [PubMed] [Google Scholar]
- 36.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer 2010;10:342–352. [DOI] [PubMed] [Google Scholar]
- 37.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci U S A 2006;103:1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang JD, Sukhova GK, Libby P, Schvartz E, Lichtenstein AH, Field SJ, Kennedy C, Madhavarapu S, Luo J, Wu D, Cantley LC. Deletion of the phosphoinositide 3-kinase p110gamma gene attenuates murine atherosclerosis. Proc Natl Acad Sci U S A 2007;104:8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siragusa M, Katare R, Meloni M, Damilano F, Hirsch E, Emanueli C, Madeddu P. Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice. Circ Res 2010;106:757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madeddu P, Kraenkel N, Barcelos LS, Siragusa M, Campagnolo P, Oikawa A, Caporali A, Herman A, Azzolino O, Barberis L, Perino A, Damilano F, Emanueli C, Hirsch E. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol 2008;28:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conte E, Gili E, Fruciano M, Korfei M, Fagone E, Iemmolo M, Lo Furno D, Giuffrida R, Crimi N, Guenther A, Vancheri C. PI3K p110gamma overexpression in idiopathic pulmonary fibrosis lung tissue and fibroblast cells: in vitro effects of its inhibition. Lab Invest 2013;93:566–576. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, Arai H, Sobue K. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation 2003;108:1746–1752. [DOI] [PubMed] [Google Scholar]
- 43.Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem 2006;281:16128–16138. [DOI] [PubMed] [Google Scholar]
- 44.Koh JS, Lieberthal W, Heydrick S, Levine JS. Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophages which acts via the phosphatidylinositol 3-kinase signaling pathway. J Clin Invest 1998;102:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Jin R, Nanda A, Yan J, Li G. Platelet PI3Kgamma Contributes to Carotid Intima-Media Thickening under Severely Reduced Flow Conditions. PloS one 2015;10:e0129265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tassi I, Cella M, Gilfillan S, Turnbull I, Diacovo TG, Penninger JM, Colonna M. p110gamma and p110delta phosphoinositide 3-kinase signaling pathways synergize to control development and functions of murine NK cells. Immunity 2007;27:214–227. [DOI] [PubMed] [Google Scholar]
- 47.Crackower MA, Oudit GY, Kozieradzki I, et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 2002;110:737–749. [DOI] [PubMed] [Google Scholar]
- 48.Patrucco E, Notte A, Barberis L, et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 2004;118:375–387. [DOI] [PubMed] [Google Scholar]
- 49.Stabile E, Zhou YF, Saji M, Castagna M, Shou M, Kinnaird TD, Baffour R, Ringel MD, Epstein SE, Fuchs S. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res 2003;93:1059–1065. [DOI] [PubMed] [Google Scholar]
- 50.Chen CN, Li YS, Yeh YT, Lee PL, Usami S, Chien S, Chiu JJ. Synergistic roles of platelet-derived growth factor-BB and interleukin-1beta in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci U S A 2006;103:2665–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caglayan E, Vantler M, Leppanen O, Gerhardt F, Mustafov L, Ten Freyhaus H, Kappert K, Odenthal M, Zimmermann WH, Tallquist MD, Rosenkranz S. Disruption of platelet-derived growth factor-dependent phosphatidylinositol 3-kinase and phospholipase Cgamma 1 activity abolishes vascular smooth muscle cell proliferation and migration and attenuates neointima formation in vivo. J Am Coll Cardiol 2011;57:2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu H, Littlewood T, Bennett M. Akt isoforms in vascular disease. Vascul Pharmacol 2015;71:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rotllan N, Wanschel AC, Fernandez-Hernando A, Salerno AG, Offermanns S, Sessa WC, Fernandez-Hernando C. Genetic Evidence Supports a Major Role for Akt1 in VSMCs During Atherogenesis. Circ Res 2015;116:1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez-Hernando C, Jozsef L, Jenkins D, Di Lorenzo A, Sessa WC. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin Y, Xie Y, Ostriker AC, et al. Opposing Actions of AKT (Protein Kinase B) Isoforms in Vascular Smooth Muscle Injury and Therapeutic Response. Arterioscler Thromb Vasc Biol 2017;37:2311–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He J, Wang H, Xiao W, Li L, Chu Q, Pan Q, Yu Y, Sun F. Mutual interaction between YAP and CREB promotes tumorigenesis in liver cancer. Hepatology 2013;58:1011–1020. [DOI] [PubMed] [Google Scholar]
- 57.Wang AB, Li HL, Zhang R, She ZG, Chen HZ, Huang Y, Liu DP, Liang CC. A20 attenuates vascular smooth muscle cell proliferation and migration through blocking PI3k/Akt singling in vitro and in vivo. J Biomed Sci 2007;14:357–371. [DOI] [PubMed] [Google Scholar]
- 58.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol 2010;185:6413–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tokunou T, Shibata R, Kai H, Ichiki T, Morisaki T, Fukuyama K, Ono H, Iino N, Masuda S, Shimokawa H, Egashira K, Imaizumi T, Takeshita A. Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells. Circulation 2003;108:1246–1252. [DOI] [PubMed] [Google Scholar]
- 60.Chava KR, Karpurapu M, Wang D, Bhanoori M, Kundumani-Sridharan V, Zhang Q, Ichiki T, Glasgow WC, Rao GN. CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol 2009;29:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molnar P, Perrault R, Louis S, Zahradka P. The cyclic AMP response element-binding protein (CREB) mediates smooth muscle cell proliferation in response to angiotensin II. J Cell Commun Signal 2014;8:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hudson C, Kimura TE, Duggirala A, Sala-Newby GB, Newby AC, Bond M. Dual Role of CREB in The Regulation of VSMC Proliferation: Mode of Activation Determines Pro- or Anti-Mitogenic Function. Sci Rep 2018;8:4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui M, Cai Z, Chu S, Sun Z, Wang X, Hu L, Yi J, Shen L, He B. Orphan Nuclear Receptor Nur77 Inhibits Angiotensin II-Induced Vascular Remodeling via Downregulation of beta-Catenin. Hypertension 2016;67:153–162. [DOI] [PubMed] [Google Scholar]
- 64.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem 2001;276:46132–46141. [DOI] [PubMed] [Google Scholar]
- 65.Garat CV, Fankell D, Erickson PF, Reusch JE, Bauer NN, McMurtry IF, Klemm DJ. Platelet-derived growth factor BB induces nuclear export and proteasomal degradation of CREB via phosphatidylinositol 3-kinase/Akt signaling in pulmonary artery smooth muscle cells. Mol Cell Biol 2006;26:4934–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.