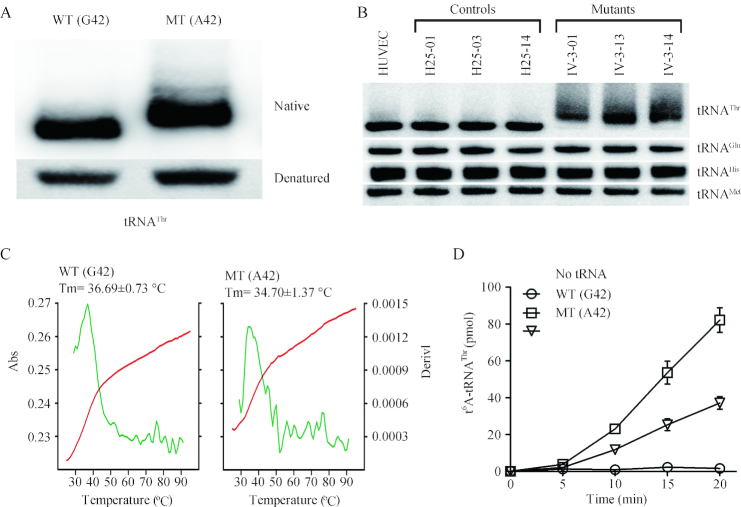
Figure 2.
In vitro analysis for the conformation, stability and t6A37 modification of tRNAThr. (A) Assessment of conformation changes by PAGE analysis under denaturing and native conditions. The transcripts of wild-type (WT) and mutated (MT) tRNAThr were electrophoresed through native or denaturing polyacrylamide gel stained with ethidium bromide. (B) Northern blot analysis of tRNAs under native conditions. Two micrograms of total mitochondrial RNA from various cell lines were electrophoresed through native polyacrylamide gel, electroblotted, and hybridized with DIG-labeled oligonucleotide probes specific for the tRNAThr, tRNAGlu, tRNAHis, and tRNAMet, respectively. (C) Melting profiles of WT and MT tRNAThr transcripts measured at 260 nm with a heating rate of 1°/min from 25 to 95° (red curves). First derivative (dA/dT) against temperature curves were shown to highlight the Tm value transitions (green curves). (D) In vitro assay for t6A37 modification. The unmodified human mitochondrial wild type (G42) and mutant (A42) tRNAThr were generated from in vitro transcription. The unmodified tRNA transcripts were incubated with yeast Sua5 and Qri7 in the presence of [14C] threonine. Samples were withdrawn and stopped after 5, 10, 15 or 20 min, respectively. The relative modification efficiency was calculated from the initial phase of the reaction. The calculations were based on three independent determinations. Graph shows the results of a representative experiment.