FIGURE 1.
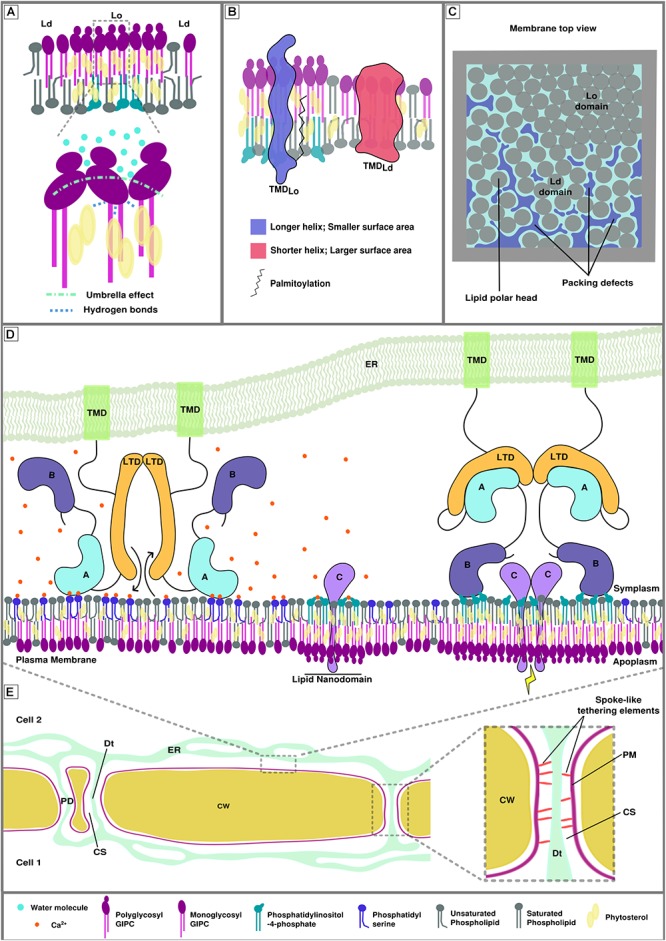
Membrane biophysical properties and lipid-protein interplay at membrane contact sites (MCSs). (A) Poly glycosylated GIPCs tend to increase the size and rigidity of phytosterol-dependent ordered membrane domains (Lo) through hydrogen bonding between the hydroxyl group of the sterols and the polarized groups of the GIPCs located at the polar/hydrophobic interface. This interaction is also favored by the umbrella effect of the big GIPCs’ polar moiety, which prevents water molecules to interact deeper into the bilayer (Grosjean et al., 2015). (B) Transmembrane protein distribution between different lipid domains relies on transmembrane length, surface area and palmitoylation (adapted from Lorent et al., 2017). (C) Representation of the lipid packing of membrane domains. Liquid ordered domain are more tightly packed than liquid disordered domains (Ld) because of the nature of the lipids and degree of their acyl chain saturation. Lipid packing defects arise in liquid disordered domains. (D) Hypothetical model of calcium-dependent regulation of protein-plasma membrane interaction at endoplasmic reticulum-plasma membrane MCS (EPCS). This hypothetical model gathers the possible interactions involving proteins, lipids and ions that could occur at MCS during signaling events. Its goal is to illustrate the complexity of lipid/protein/ion interactions. The protein illustrated here represents a lipid transfer protein/tether element that specifically locates to EPCS upon homodimerization. Left. In presence of calcium, domain A is able to interact with phosphatidylserine, the inter-membrane gap is reduced, allowing the exchange of lipids by the lipid transfer domains (LTDs). Domain B cannot interact with the phosphatidylinositol phosphates of the lipid nanodomains as they are shielded by the calcium ions (Middle). Right. In the absence of calcium, domain A is released from the membrane, increasing the inter-membrane gap, and binds to the LTD, inhibiting lipid exchange between organelles. Domain B docks onto the lipid nanodomains via electrostatic interactions with anionic PIPs and leads to the formation of bigger lipid domains where protein C can interact with one another and initiate/relay a signal. There are two main domain types allowing peripheral binding of proteins, the anionic lipid and/or calcium-dependent C2 domains (such as domain A in this figure) and the anionic lipid dependent PH domains (such as domain B in this figure). (E) Schematic view of plant cell-to-cell junction showing the cell wall (CW), the endoplasmic reticulum (ER) network, plasma membrane (PM) and several plasmodesmata (PD). The right insert shows the PD ultrastructure. The close vicinity between the PM and the desmotubule (Dt;a lumen-free tubule of ER), connected by spoke-like tethering elements, leaves a small inter-membrane gap between the two membranes, called the cytoplasmic sleeve (CS).
