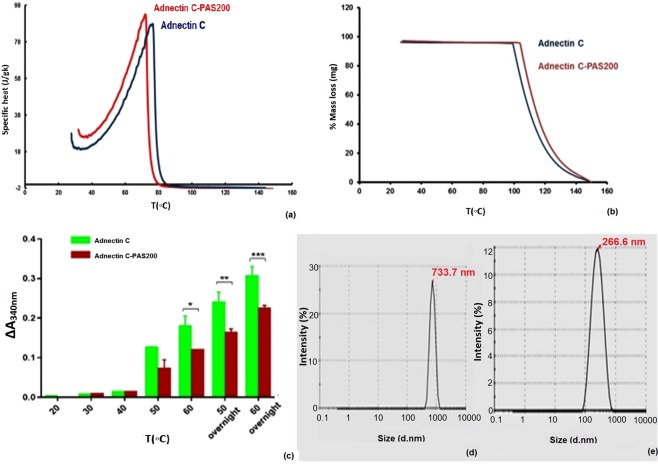
Figure 4.
Thermo-analysis and protein aggregation assessment under different thermal and freeze-thaw conditions: (a) DSC thermograms; (b) TG curves of two-state unfolding PASylated Adnectin C and native protein. Samples were prepared in phosphate buffer at pH 6.8 and analyzed in inert atmosphere at a heating rate of 2 °C/min. A negligible shift in both thermograms was observed for PASylated Adnectin C relative to the native protein; (c) aggregation of PASylated Adnectin C and native protein after incubation at the defined temperatures and incubations times. A large increase in A340nm after heating indicates lower thermal stability. Data are represented as mean ± SD (three replicates). Asterisks denote the significance levels (*p < 0.05, **p < 0.01, and ***p < 0.001); (d,e) DLS of freeze-thaw effect on protein aggregation of the recombinant proteins. Peak shifts were observed for: (d) the native (96.35 nm to 733.7 nm) and (e) PASylated protein (192.3 to 266.6 nm). Lower aggregated forms for PASylated were observed.