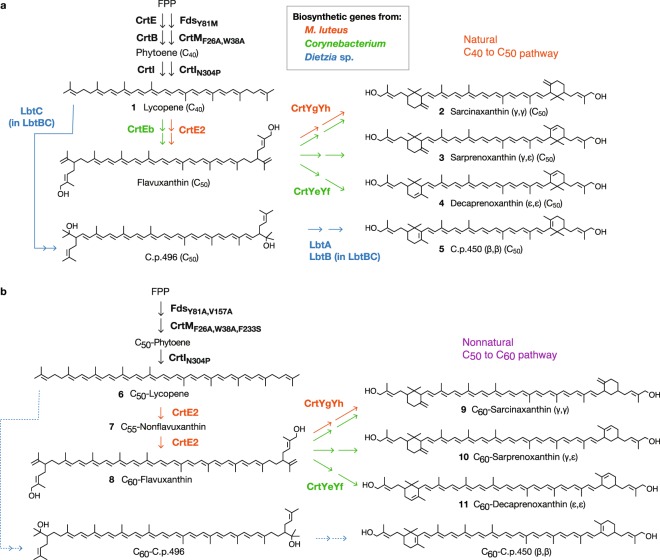
Figure 1.
C5-elongation and cyclization pathways for natural (C50) and nonnatural (C60) carotenoids. (a) Natural C40-to-C50 carotenoid pathway. Using lycopene (C40) as a substrate, the lycopene elongases CrtEb from Corynebacterium and CrtE2 from M. luteus attaches two isoprene units to C40. The resulting flavuxanthin (C50) is then cyclized by the γ- and/or ε-cyclases CrtYe/Yf (Corynebacterium) or CrtYg/Yh (M. luteus) to produce sarcinaxanthin, sarprenoxanthin, or decaprenoxanthin (all C50). LbtABC genes from Dietzia sp. CQ4 produces the β,β-cyclic C50 carotenoid C.p.450 via the independent intermediate C.p.496. (b) Nonnatural C50-to-C60 carotenoid pathway construction. Combined expression of Corynebacterium and M. luteus elongases and cyclases resulted in the conversion of laboratory-generated C50-lycopene to C60 carotenoids with γ and/or ε-cyclic ends. We could not obtain C60 counterparts of β-end C50 carotenoids (indicated in arrows with dashed lines).