Figure 1.
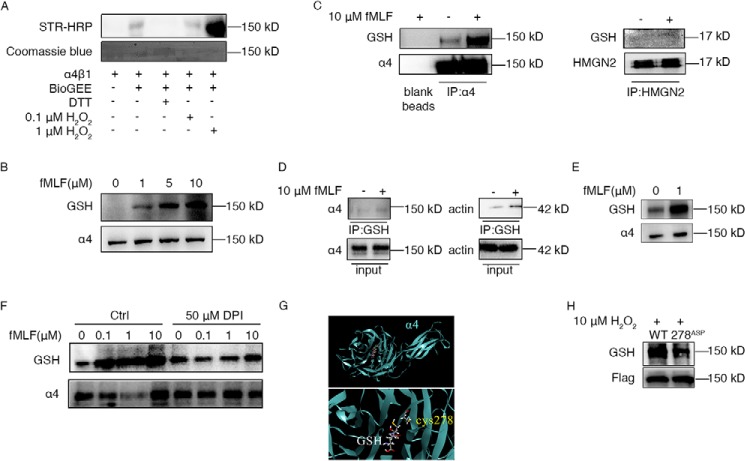
ROS induced elevated α4 integrin glutathionylation in neutrophils. A, recombined integrin α4β1 protein was treated with biotinylated GSH (BioGEE), DTT, and H2O2 at the indicated concentrations for 30 min. Then the solutions were subjected to immunoblotting to detect BioGEE-modified α4 with streptavidin–horseradish peroxidase (STR-HRP). Total protein loading was evaluated with Coomassie blue dye. Every treatment used 0.5 μg protein. B, neutrophil-like dHL60 cells were treated with the indicated concentrations of fMLF for 5 min. Cells were harvested and subjected to immunoblotting for GSH and α4. C, dHL60 cells were left untreated or stimulated with 10 μm fMLF for 5 min. Whole-cell lysates were then subjected to α4 immunoprecipitation (IP) followed by immunoblotting for GSH-modified α4. Blank agarose was tested as a negative control. HMGN2 (without cysteine) antibody–induced pulldown was used as a control. D, dHL60 cells were left untreated or stimulated with 10 μm fMLF for 5 min. Whole-cell lysates were then subjected to GSH IP followed by immunoblotting for α4. Actin antibody was used as a control. E, murine neutrophils were left untreated or stimulated with 1 μm fMLF for 5 min. Whole-cell lysates were subjected to immunoblotting for GSH and α4. F, dHL60 cells were pretreated or not pretreated with 50 μm DPI for 30 min. Then both groups of dHL60 cells were stimulated with the indicated concentrations of fMLF for 5 min. Cells were harvested and subjected to immunoblotting for GSH and α4. G, GSH was docked into the α4 integrin crystal structure in complex with the α4 integrin β-propeller and thigh domain crystal structure (PDB code 4IRZ). The dominant pose of 10 docking runs is represented as sticks, and interacting cysteine is represented as sticks. H, dHL60 cells were transfected with constructs expressing either the WT or the 278Asp mutant form of FLAG–α4 integrin. These dHL60 cells were stimulated with 10 μm H2O2 for 5 min. Whole-cell lysates were then subjected to FLAG IP followed by immunoblotting for GSH-modified FLAG-α4. All data are representative of at least three separate experiments.