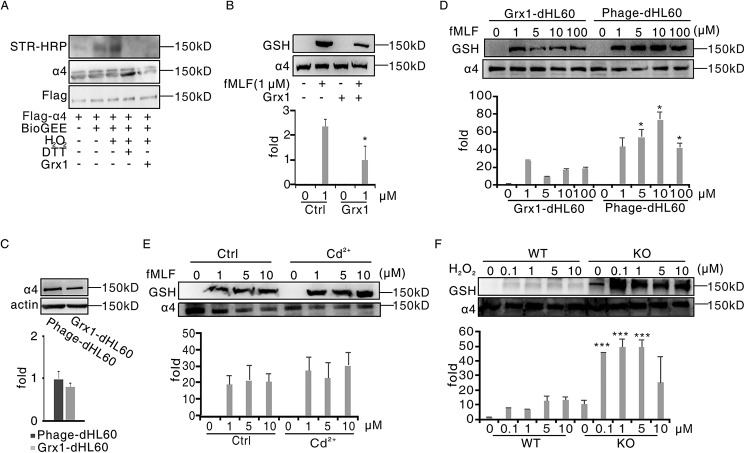
Figure 3.
Grx1 physiologically catalyzed α4 integrin deglutathionylation. A, HEK293T cells were transfected with plasmids encoding FLAG-α4. After 48 h, cells were harvested and pulled down through anti-FLAG–agarose beads. Beads were treated with BioGEE and H2O2 at the indicated concentrations for 15 min. After adding 1 μg of Grx1 protein and being incubated for another 15 min, the solutions were subjected to immunoblot analysis for BioGEE-modified α4 with streptavidin–horseradish peroxidase (STR-HRP), and total α4 loading was evaluated with FLAG and α4 antibodies. B, dHL60 cells were preincubated with 1 μg of Grx1 protein or not for 30 min and then treated with 1 μm fMLF for 5 min. Cells were harvested and subjected to immunoblot analysis for GSH and α4. C, control dHL60 cells (Phage-dHL60) and Grx1-overexpressing dHL60 cells (Grx1-dHL60) were harvested and subjected to immunoblot analysis for α4 and actin detection. D, phage-dHL60 and Grx1-dHL60 cells were treated with the indicated concentrations of H2O2 for 5 min. Cells were harvested and subjected to SDS-PAGE followed by immunoblot analysis for GSH and α4. E, Grx1-dHL60 cells were pretreated with 2 mm Grx1 inhibitor Cd2+ or not for 30 min and then stimulated with the indicated concentration of H2O2 for 5 min. Cells were harvested and subjected to SDS-PAGE followed by immunoblot analysis for GSH and α4. F, WT and Grx1−/− mouse neutrophils were treated with the indicated concentrations of H2O2 for 5 min. Cells were harvested and subjected to SDS-PAGE followed by immunoblot analysis for GSH and α4. All data are representative of at least three separate experiments.