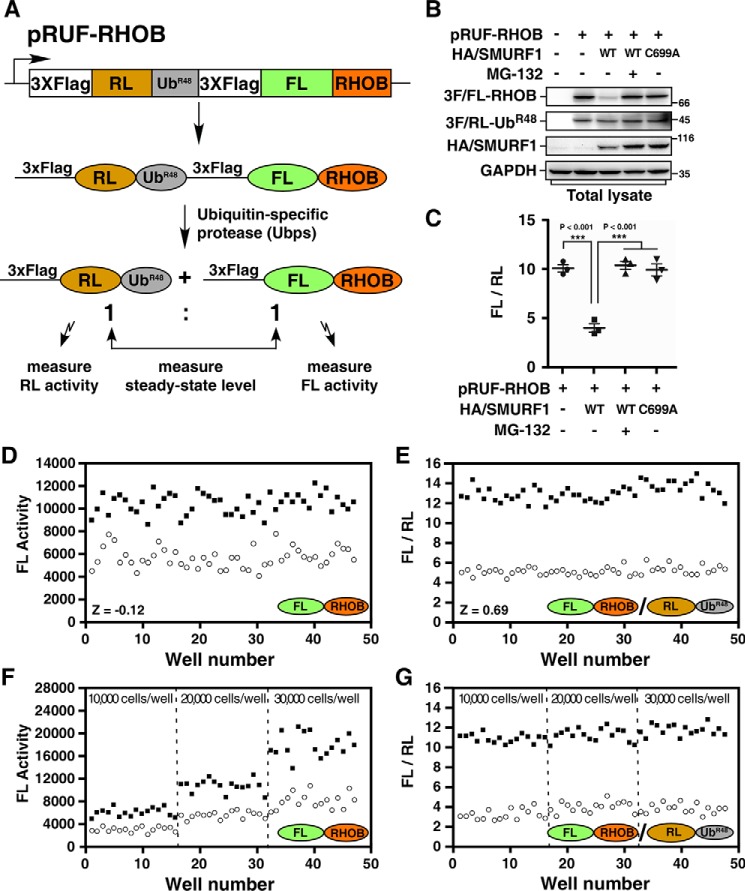
Figure 1.
Cell-based high-throughput URT-Dual-Luciferase Assay system for SMURF1. A, a schematic of the pRUF-RHOB construct. A fusion protein comprised of triple FLAG-tagged RL, UbR48 moiety, triple FLAG-tagged FL, and RHOB is cleaved in vivo by Ubps at the UbR48-RHOB junction to yield equimolars of triple FLAG-tagged RL-UbR48 and triple FLAG-tagged FL-RHOB. The triple FLAG-tagged FL-RHOB is a substrate of SMURF1 and will be degraded in the presence of SMURF1. B, analysis of the steady-state levels of FL-RHOB by immunoblotting. HEK293T cells were transfected with pRUF-RHOB and HA-tagged SMURF1 (HA/SMURF1), WT, or catalytically inactive mutant C699A, as indicated. After overnight treatment with or without 5 μm MG-132, total cell lysates were subjected to immunoblotting using indicated antibodies to determine the steady-state protein levels. C, luciferase assay of FL-RHOB. HEK293T cells were transfected with pRUF-RHOB and HA/SMURF1 WT or C699A, and treated with or without 5 μm MG-132 as in (B) and then applied to Dual-Glo Luciferase Assay to measure the activities of FL and RL. Results were plotted as the ratio of FL activity to RL activity (FL/RL). D and E, evaluation of the screening system. HEK293T cells co-transfected with pRUF-RHOB and WT HA/SMURF1 were treated overnight with DMSO (open circles) and 5 μm MG-132 (filled squares) as negative or positive controls, respectively. Luciferase activities were measured and plotted using either FL activity alone (D) or the FL/RL ratio (E). F and G, the URT system effectively corrects variation of cell numbers. HEK293T cells co-transfected with pRUF-RHOB and WT HA/SMURF1 were seeded with varying number of cells as indicated and treated with DMSO or MG-132 as in (D). Luciferase activities were measured and plotted as FL alone (F) or FL/RL (G).