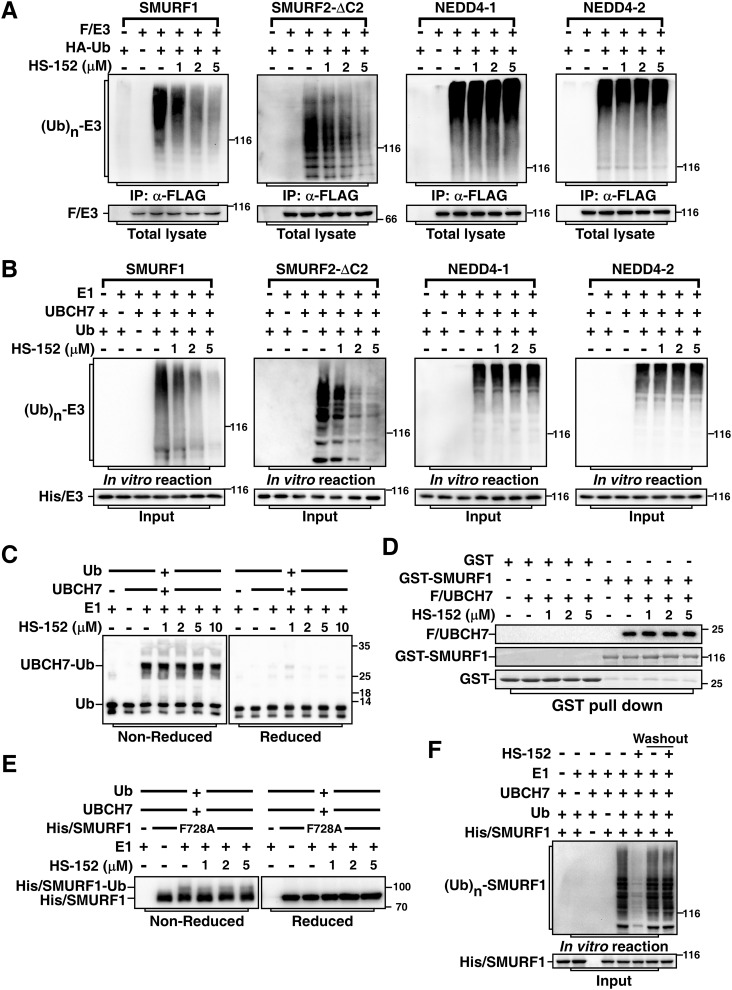
Figure 5.
HS-152 inhibits catalytic activities of SMURFs. A, HS-152 inhibits auto-ubiquitination of SMURF1 and SMURF2-ΔC2 in cells. HEK293T cells were transfected with HA-tagged ubiquitin (HA/Ub) and FLAG-tagged SMURF1, SMURF2-ΔC2, NEDD4–1, or NEDD4–2 as indicated. The cells were treated 3 h with 40 μm MG-132 and then subjected to anti-FLAG immunoprecipitation (IP) followed by immunoblotting assay to detect ubiquitin-conjugated E3s ((Ub)n-E3) using anti-HA antibody. B, HS-152 inhibits auto-ubiquitination of SMURF1 and SMURF2-ΔC2 in vitro. Purified SMURF1, SMURF2-ΔC2, NEDD4–1, and NEDD4–2 were subjected to an in vitro auto-ubiquitination assay in the absence or presence of HS-152 at the indicated concentrations and ubiquitin-conjugated E3s ((Ub)n-E3) were detected by immunoblotting with anti-ubiquitin antibody. C, HS-152 does not inhibit E2 UBCH7 ubiquitin conjugation. E2 UBCH7 conjugation by E1 enzyme was conducted with varying concentrations of HS-152 as indicated. Reactions were stopped in nonreducing SDS sample buffer and then were applied to immunoblotting under nonreducing condition (left panel), or were reduced using DTT prior to SDS-PAGE (right panel). Ubiquitin and ubiquitin conjugated to UBCH7 via the reductant-sensitive thioester bond are indicated. D, HS-152 does not affect binding of E2 to Smurf1. Purified Smurf1 and UBCH7 were subjected to GST pulldown assay in the absence or presence of indicated amount of HS-152. E, HS-152 does not affect formation of ubiquitin-thioester intermediate in vitro. Purified Smurf1-F728A mutant was subjected to an in vitro auto-ubiquitination assay in the absence or presence of indicated amount HS-152. Reactions were stopped as in (C) to examine the thioester bond formation. F, HS-152 is a reversible inhibitor of SMURF1. Purified SMURF1 was incubated with 5 μm HS-152 for 20 min and then carried out with or without washing three times, as indicated, before subjected to an in vitro auto-ubiquitination assay.