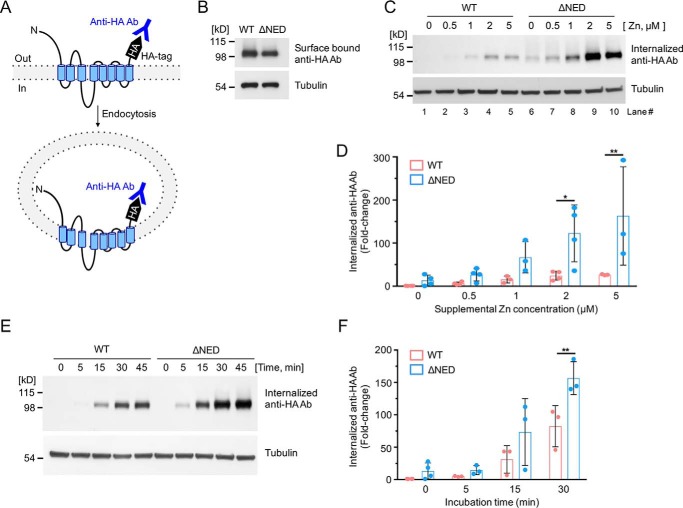
Figure 2.
Sensitivity of the mZIP4 N-terminal extracellular domain mutant to zinc-stimulated endocytosis. A, schematic illustration of mZIP4 showing the extracellular orientation of the HA tag, which permits labeling of mZIP4 by anti-HA antibodies at the plasma membrane, and measuring mZIP4 endocytosis. Zinc-stimulated endocytosis of WT and ΔNED-mZIP4-HA proteins was analyzed by measuring internalized anti-HA antibodies. B, WT-mZIP4-HA and ΔNED-mZIP4-HA levels at the cell surface were determined by incubating HEK293 cells in Chelex 100-treated medium. C, cells were preincubated with Chelex-treated medium for 3 h to increase surface levels of mZIP4-HA. Immunoblots were used to detect levels of internalized anti-HA antibodies endocytosed in HEK/WT-mZIP4-HA or HEK/ΔNED-mZIP4-HA cells for 5 min in either Chelex-treated medium (lanes 1 and 6) or Chelex-treated medium containing the indicated zinc concentrations. Tubulin levels shown in the lower panel demonstrate equal protein loading. D, quantification of zinc dose-dependent internalization of anti-HA antibodies in HEK293 cells expressing WT or ΔNED-mZIP4-HA (C). E, uptake of anti-HA antibodies by HEK/WT-mZIP4-HA or HEK/ΔNED-mZIP4-HA cells was assessed for the indicated time courses. Internalized anti-HA antibodies were detected by Western blot analysis as described under “Experimental procedures.” Tubulin is shown as a loading control. F, quantification of the internalization of anti-HA antibodies over time (Fig. 2E). Error bars in D and F indicate relative fold-changes compared with basal conditions of WT mZIP4-HA (mean ± S.D., n = 3–4). Statistics: two-way ANOVA, Sidak's post hoc test (*, p < 0.05, and **, p < 0.01).