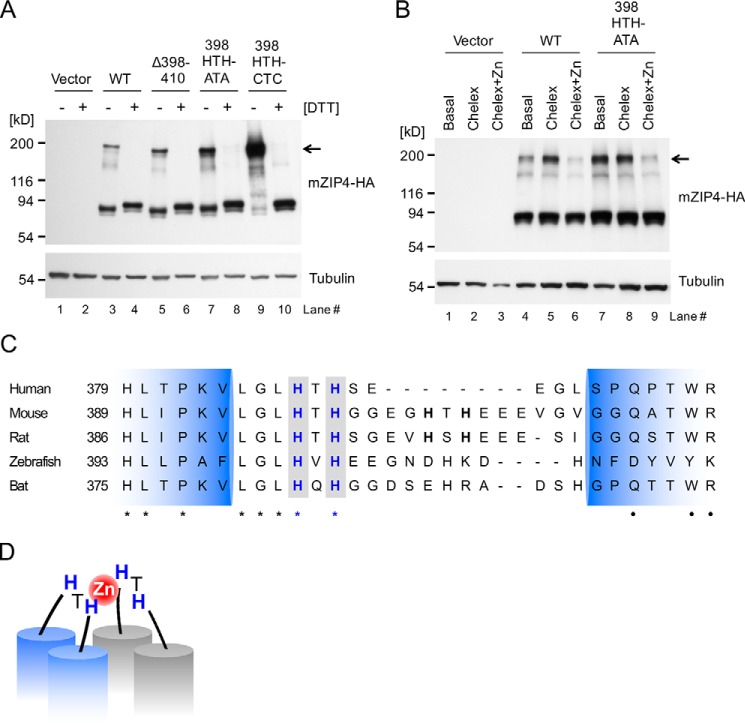
Figure 7.
mZIP4 exists as homodimers at the cell surface. A, HEK293 cells stably expressing WT and mutant mZIP4 proteins were analyzed by immunoblotting with (+) or without (−) DTT. Arrow indicates dimeric species of mZIP4 proteins. B, HEK293 cells stably expressing WT and mutant mZIP4-HA proteins were preincubated with basal medium, Chelex-treated medium, or Chelex-treated medium containing 10 μm zinc for 3 h to differentiate surface levels of the proteins, and then the cells were incubated with the membrane impermeable chemical cross-linker Sulfo-EGS at 37 °C to cross-link cell-surface proteins followed by quenching with Tris-HCl buffer, resolution via SDS-PAGE, and immunoblotting with anti-HA antibody. The expected molecular weight of the dimeric species of mZIP4 is indicated by the arrow. C, multiple sequence alignment of ZIP4 orthologs in mammals in the region of the first extracellular loop between the predicted TMD 2 and 3. NCBI reference sequence codes are: NP_570901.2 for human, NP_082340.1 for mouse, A0JPN2.1 for rat, XP_017207743.1 for zebrafish, and ELK11751.1 for bat. Conserved HXH motifs are shown in bold blue font with a gray background. Identical amino acids are indicated by asterisks (*), and high similarity amino acids are indicated by dots (●). Extracellular loop regions (389–420 amino acid residues) between TMD 2 and 3 of mZIP4 were predicted using TMHMM. D, a proposed model of zinc binding to the first extracellular loop of the ZIP4 homodimer. Histidine residues in the 398HTH motif in the first extracellular loops may coordinate a zinc ion and provide a high zinc sensitivity in zinc-stimulated endocytosis of ZIP4 proteins.