Figure 1.
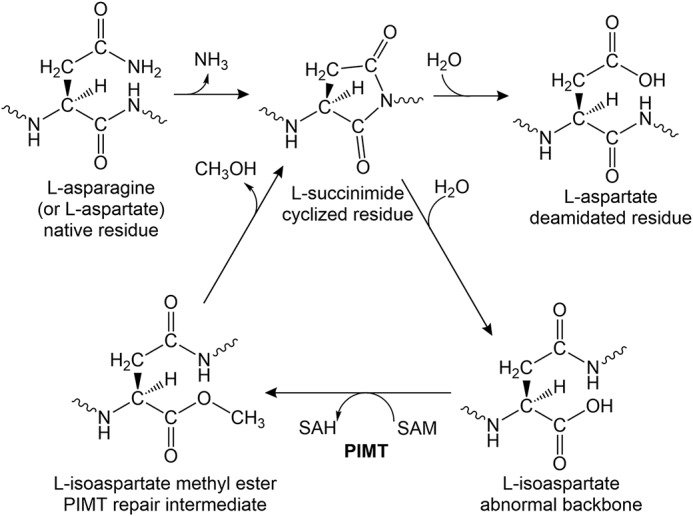
The pathway of l-isoaspartyl repair by PIMTs. l-Aspartyl and l-asparaginyl residues can spontaneously cyclize to l-succinimides. The succinimides undergo spontaneous hydrolysis resulting in a regeneration of l-aspartyl residues with a frequency of ∼25%, but this is also accompanied by the generation of abnormal l-isoaspartyl residues with a frequency of ∼75%. l-Isoaspartates introduce kinks into the backbone of proteins, which can compromise structure and activity. The repair enzyme PIMT transfers a methyl group from SAM to the side chain of l-isoaspartate, forming l-isoaspartate methyl ester. The methyl ester is unstable and spontaneously converts back to l-succinimide, which again hydrolyzes to form a combination of l-aspartyl and l-isoaspartyl residues.
