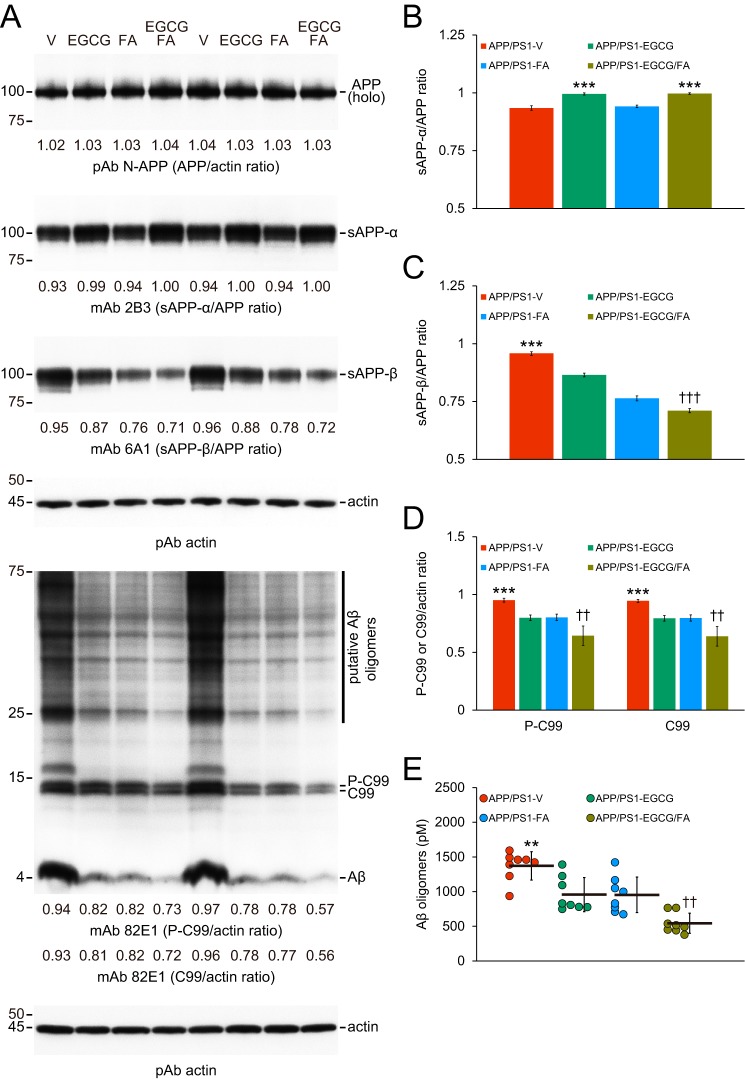
Figure 5.
Combined therapy promotes nonamyloidogenic and inhibits amyloidogenic APP cleavage. Data were obtained from APP/PS1 mice that received vehicle (APP/PS1-V, n = 8), EGCG (APP/PS1-EGCG, n = 8), FA (APP/PS1-FA, n = 8), or EGCG plus FA (APP/PS1-EGCG/FA, n = 8) for 3 months starting at 12 months of age. Western blottings included each mouse (n = 8 per group), and quantitative data were averaged. Ten μg of protein from each sample was equally loaded per lane. Data for B–E are presented as standard deviations of the means. A, Western blots are shown using N-terminal APP polyclonal antibody (pAb N-APP), C-terminal anti-sAPP-β-sw mAb (mAb 6A1), and sAPP-α mAb (mAb 2B3). Western blots are also shown using N-terminal β-amyloid(1–17) (Aβ) mAb (mAb 82E1), which detects amyloidogenic APP cleavage fragments, including Aβ monomer and oligomers as well as phospho-C99 (P-C99) and nonphospho-C99 (C99). Actin is included as a loading control, and densitometry values are indicated below each lane. B–D, densitometry data are shown for ratios of sAPP-α or sAPP-β to APP as well as P-C99 or C-99 to actin. E, abundance of (N) 82E1 Aβ oligomers in the detergent-soluble brain homogenate fraction (measured by sandwich ELISA) are shown. Statistical comparisons for B–C and E are between-groups. Statistical comparisons for D are within each protein and between-groups. B, ***, p < 0.001 for APP/PS1-EGCG and APP/PS1-EGCG/FA mice versus APP/PS1-V and APP/PS1-FA mice; C–E, **, p < 0.01; ***, p < 0.001 for APP/PS1-V versus the other treated mice; ††, p < 0.01; †††, p < 0.001 for APP/PS1-EGCG/FA versus APP/PS1-EGCG or APP/PS1-FA mice. V, vehicle.