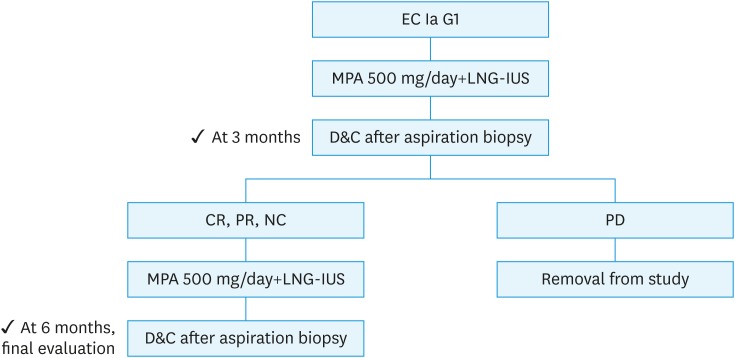
Fig. 1. Study design.
CR, complete response (defined as absence of any hyperplastic or cancerous lesion); D&C, dilation and curettage; EC, endometrial carcinoma; G1, grade 1; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate; NC, no change (defined as residual lesion without degeneration or atrophy of endometrial glands); PD, progressive disease (defined as appearance of grade 2 or 3 endometrial carcinoma); PR, partial response (defined as residual lesion with degeneration and atrophy of endometrial glands).