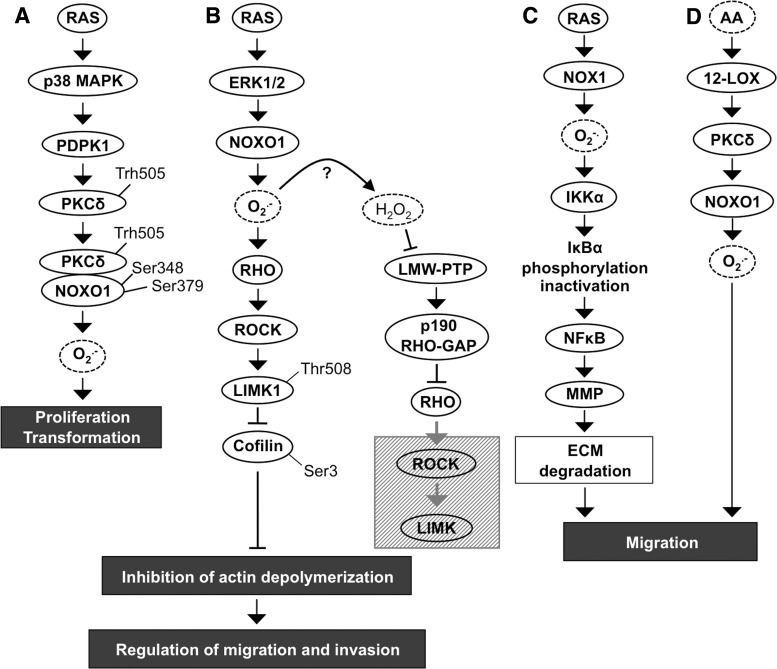
FIG. 2.
RAS induces the proliferation and migration of cancer cells via NOX1. (A) The RAS-p38MAPK signaling pathway induces PKCδ phosphorylation at Thr505, which causes consequent PKCδ-NOXO1 dimerization and phosphorylation of NOXO1 at Ser348 and Ser379. NADPH oxidase NOX1 produces O2•− thereby stimulating cancer cell migration. (B) RAS-ERK1/2 induced NOXO1 activation and increased O2•−-stimulated signaling downstream to the RHO-ROCK-LIMK1 pathway that then inhibits cofilin by phosphorylation at Ser3 and consequently impacts actin depolymerization. H2O2 produced after activation of NOXO1 may inactivate phosphotyrosine phosphatase LMW-PTP. Consequent increased expression of p190 RHO GAP enhances GTP removal from RHO small GTPase downregulating the downstream ROCK-LIMK1 pathway. (C) RAS-induced signaling through IKKα-NFκB induces local cancer cell migration by MMP activation and ECM degradation. (D) Migration is also stimulated by arachidonic acid and 12-lipoxygenase-induced PKCδ signaling that activates NOXO1 and increases O2•− production. ECM, extracellular matrix; GAP, small GTPase activator protein; IKKα, inhibitor of nuclear factor kappa-B kinase subunit α; LIMK, LIM kinase; LMW-PTP, low-molecular-weight phosphotyrosine phosphatase; MMP, matrix metalloproteinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PTP, protein tyrosine phosphatase; ROCK, RHO-associated, coiled-coil-containing protein kinase.