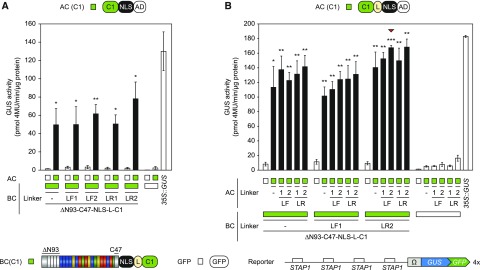
Figure 4.
Integration of linkers within the AC- and BC-scaffold. Modifications are visualized by schematic representations above and below the diagrams (Linkers: F, flexible, R, rigid). The diagrams show the results of the GUS assays after Agrobacterium-mediated transient expression of indicated split-TALE components (BC, AC) and the reporter construct (4×STAP-Ω-GUS-GFP) in N. benthamiana leaves. The color code indicates corresponding split-TALE constructs (C1, green boxes) or free GFP (35S::GFP, white boxes). Free GFP was used to monitor background activity of the single BC and AC, respectively. 35S::GUS serves as the positive control. White bars are for assays where only one component of the sTALE, either BC or AC, was expressed. Black bars are for assays where both functional components were expressed. The identity of the expressed component is indicated below the graph by green boxes or squares. A, Linker-containing BCs were combined with ACs without linkers. Flexible linkers within the BC-scaffold showed no clear effect, whereas the rigid linker 2 (LR2) led to slightly increased split TALE activity. B, Linker-containing BCs were combined with linker-containing ACs. Rigid linkers within the AC-scaffold showed a trend to positive effects on split-TALE activity. The combination of BC(LR2) and AC(LF2) possessed the best signal-to-noise ratio (indicated by a red triangle). Error bars represent sd (sd) of three biological replicates. Asterisks indicate a significant difference in activity of the same BC tested with free GFP (white box; Student’s t test; *P-value ≤ 0.05, **P-value ≤ 0.01, ***P-value ≤ 0.001). Experiments were performed three times with similar results. All raw data are available in the Supplemental Data.