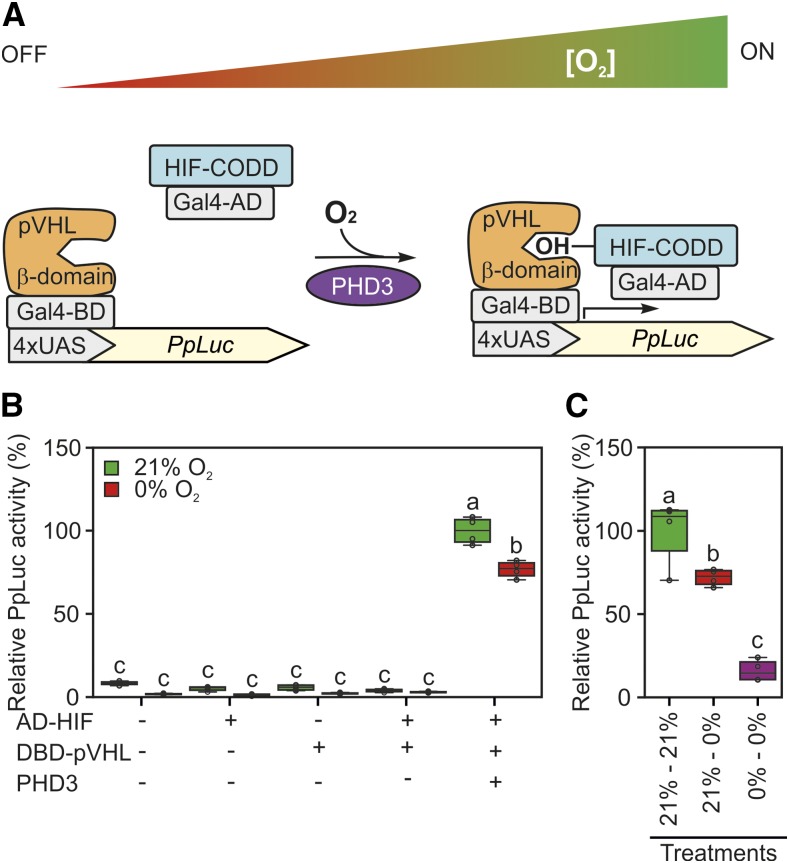
Figure 2.
Schematization and testing of a synthetic oxygen sensor device based on the mammalian hypoxia sensing. A, Conceptual working mechanism of the device dependent on the environmental oxygen concentration. Human HIF1-α or pVHL fragments (HIF-CODD and β-domain) were fused in frame with the Gal4 AD or DBD, respectively. In the presence of oxygen, AD-HIF is hydroxylated by PHD3 and dimerizes with DBD-pVHL to induce the expression of a firefly luciferase reporter (PpLuc) controlled by a 4xUAS promoter. B, Sensor output in Arabidopsis protoplasts subjected to 18-h-long anoxia (0% oxygen) or normoxia (21% [v/v] oxygen) 12 h after transfection with plasmids bearing the modules depicted in A. Firefly luciferase activity was normalized to that of a sea pansy luciferase, coexpressed under the control of a constitutive CaMV 35S promoter. The average aerobic value produced by the full device (equipped with reporter, sensory, and effector modules) was set to 100%. C, Comparison of sensor responsiveness to an 18-h-long anoxic treatment applied either 12 h after protoplast transfection (21%–0%) or immediately after it (0%–0%). In the box plots, dots represent single data points, the black line marks the median, and the box indicates the interquartile range (IQR). Whiskers extend to data points that are lower than 1.5× IQR away from the box extremities. Different letters indicate statistically significant differences (P ≤ 0.05) calculated from two-way ANOVA followed by Tukey’s posttest.