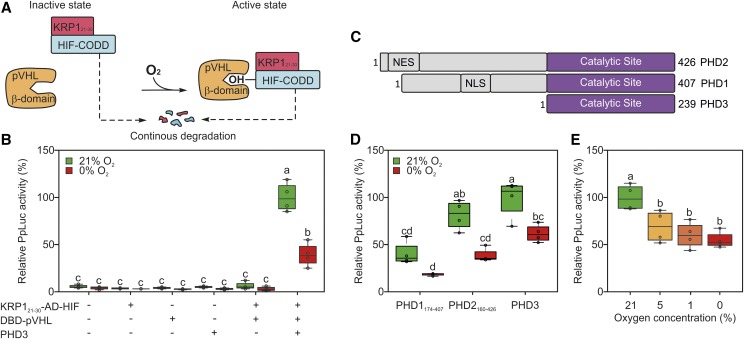
Figure 3.
Engineering of a destabilized version of the AD-HIF protein. A, To increase HIF-CODD turnover, the AD-HIF effector module was modified with an additional destabilizing domain (KRP121-30), promoting its recycling through the 26S proteasome. For the sake of simplicity, the Gal4 domains and the reporter construct are not shown in the schematic. B, Sensor output in an Arabidopsis protoplast transiently transformed with reporter, sensory, and effector plasmids and subjected to 18 h of anoxia 12 h after transfection. C, Structures of the three known human PHD isoforms, according to the information related to the Uniprot accessions Q96KS0 (PHD1), Q9GZT9 (PHD2), and S5Q9G2 (PHD3). NES stands for nuclear export signal and NLS for nuclear localization signal. D, Sensor output in protoplasts transfected with one of the three known human PHD isoforms. Effector and reporter modules were provided equally in all transformations. E, Arabidopsis protoplasts were incubated under a range of oxygen concentrations, spanning from complete anoxia to aerobic levels. In the box plots, dots represent single data points, the black line marks the median, and the box indicates the interquartile range (IQR). Whiskers extend to data points that are lower than 1.5× IQR away from the box extremities. Different letters indicate statistically significant differences (P ≤ 0.05) among means, as assessed by two-way ANOVA (in B and D) or one-way ANOVA (in E) followed by Tukey’s posttest to correct for multiple comparisons.