Abstract
Visceral adipose tissue (VAT)—fat stored deep in the abdominal cavity that surrounds vital organs—is associated with a variety of chronic health conditions. Computed tomography and magnetic resonance imaging are the gold standards to quantify VAT. However, the high cost, limited accessibility, and potential exposure to radiation limit the use of these imaging modalities. In this commentary, we review the application of a previously validated regression equation that estimates anthropometrically predicted VAT (apVAT) to explain variance in blood-based biomarkers and predict mortality in a large sample of adults. In our first study (Brown et al. 2018 Eur J Nutr; doi:10.1007/s00394-016-1308-8), apVAT accounted for more variance in biomarkers of glucose homeostasis, inflammation, and lipid metabolism, than body mass index (BMI), waist circumference (WC), or the combination of BMI + WC. In our second study (Brown et al. 2017 Am J Hum Biol; doi:10.1002/ajhb.22898), compared with BMI, WC, and BMI + WC, apVAT more accurately predicted mortality from all causes, cardiovascular disease, and cancer. These studies demonstrate that apVAT can be used in clinical practice and in clinical nutrition and metabolism research when imaging modalities to quantify VAT may not be feasible.
Keywords: Obesity, insulin resistance, inflammation, body composition, cohort study
Comment on: Brown JC, Harhay MO, Harhay MN. Anthropometrically predicted visceral adipose tissue and blood-based biomarkers: a cross-sectional analysis. Eur J Nutr. 2018; 57:191-198. doi: 10.1007/s00394-016-1308-8.
Commentary
The accurate measurement of body composition is an essential component of nutritional and metabolic phenotyping. Body composition science has refined our ability to determine the relationship of body mass with health outcomes by providing distinct measures of adipose and lean muscle tissues.1 Visceral adipose tissue (VAT)—fat stored deep in the abdominal cavity that surrounds vital organs—is associated with a variety of chronic health conditions, such as type 2 diabetes, cardiovascular disease, multiple forms of cancer, and premature mortality.2 Computed tomography and magnetic resonance imaging are the gold standards to quantify VAT.3 However, the high cost, limited accessibility, and potential exposure to radiation limit the use of these imaging modalities in clinical practice and in large intervention or epidemiologic studies. In contrast, anthropometric measures that estimate VAT have the potential to enhance clinical care and clinical research by providing an additional non-invasive tool to characterize cardiovascular, metabolic, and oncologic health risks.
Waist circumference (WC) is often used as a proxy measure of VAT. However, WC is more strongly correlated with body mass index (BMI; R2 = 0.86) and fat mass (R2 = 0.85), than it is with VAT (R2 = 0.59).4 This is because WC cannot differentiate VAT from the less deleterious subcutaneous adipose tissue that is also stored in the abdomen. Several equations that simultaneously integrate multiple anthropometric measures, such as height, weight, and circumferences of the waist and thigh, have been developed to estimate VAT. These equations have been validated against various imaging modalities and strongly correlate with VAT (R2 = 0.80-0.84).5–7 However, it was unknown whether these estimates of anthropometrically predicted VAT (apVAT) were associated with blood-based biomarkers of cardiometabolic health.8 Furthermore, it was unknown whether apVAT was a predictor of long-term clinical outcomes, such as mortality from cardiovascular disease and cancer.9 Therefore, we sought to understand the clinical and research importance of apVAT by testing these above-described hypotheses. Moreover, we sought to determine whether apVAT better explained variance in metabolic biomarkers and more accurately prognosticated mortality when compared with other common anthropometric measures. If these hypotheses were supported, it would offer additional validity for the use of apVAT in clinical and research settings. Herein, we briefly summarize the results of these studies.8,9
We tested the hypothesis that apVAT is correlated with and accounts for more variance in biomarkers of glucose homeostasis, inflammation, and lipid metabolism than BMI, WC, or their combination (BMI + WC). The dysregulation of these biomarkers is hypothesized as some of the physiologic mechanisms that may underpin the health risks associated with being overweight or obese.2 Participants in this cross-sectional analysis included 10 624 white men and women who participated in the Third National Health and Nutrition Examination Survey (NHANES III; 1988-1994).8 The average age of participants was 44.6 ± 0.3 years, and 48.2% were men. Bootstrapped linear regression models were used to calculate and compare the proportion of variance (R2) in biomarkers that was explained by apVAT, BMI, WC, and BMI + WC. apVAT was correlated with all biomarker measures. apVAT explained 12.4% to 16.1% of the variance in measures of glucose homeostasis (eg fasting plasma glucose and hemoglobin A1c), 4.6% to 8.2% of the variance in measures of inflammation (eg C-reactive protein and fibrinogen), and 4.5% to 17.3% of the variance in measures of lipid metabolism (eg apolipoprotein B, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and total cholesterol).8 apVAT explained statistically significantly more variance in nearly every biomarker when compared with BMI, WC, and BMI + WC.8 The two exceptions were C-reactive protein and triglyceride concentrations, where WC and BMI + WC explained a similar amount of variance in these biomarkers as apVAT. Finally, apVAT more accurately predicted the presence of impaired fasting glucose and the metabolic syndrome than all other anthropometric measures. In conclusion, this study demonstrated that apVAT accounts for more variance in biomarkers related to glucose homeostasis, inflammation, and lipid metabolism than the more traditional anthropometric metrics of BMI, WC, and BMI + WC.8
Given evidence that apVAT was associated with metabolic biomarkers, we tested the hypothesis that apVAT is also associated with mortality and more accurately predicts mortality than BMI, WC, and BMI + WC. Participants in this longitudinal analysis included the 10 624 white men and women who were included in the above-described cross-sectional analysis. After a median of 18.8 years of follow-up, 3531 deaths from all causes were observed; 1151 and 741 deaths were attributable to cardiovascular disease and cancer, respectively. A graded linear dose–response relationship was observed between apVAT and mortality from all causes (P for trend < .001), cardiovascular disease (P for trend < .001), and cancer (P for trend = .007).9 Compared with participants in the lowest quintile of apVAT, those in the highest quintile were 2.19-, 2.39-, and 2.70 times more likely to die from all causes, cardiovascular disease, and cancer, respectively.9 These associations were independent of various prognostic factors such as age, sex, smoking status, dietary patterns, and physical activity. apVAT more accurately predicted mortality from all causes, cardiovascular disease, and cancer, as compared with BMI (22.0%-27.5% improved accuracy), WC (13.6%-18.2%), and BMI + WC (8.0%-11.5%; all Ps < .001).9 In conclusion, this study demonstrated that apVAT is associated with mortality and more accurately predicts mortality than BMI, WC, and BMI + WC.9
The main findings from these studies demonstrate the potential value that apVAT may have in nutritional and metabolic phenotyping. The prediction of apVAT requires the measurement of height, body mass, and circumferences of the waist and thigh (and sex and age).7 apVAT offers a low-cost, non-invasive, accessible measure that can be used in routine clinical practice and in clinical trials or epidemiologic studies, thereby circumventing many of the most common barriers associated with other VAT measurement modalities such as computed tomography or magnetic resonance imaging.3 However, there are several limitations to this line of research. The primary limitation is that the regression equation that was used in our study to estimate apVAT has only been validated in a white population. It is recognized that body composition varies among different racial and ethnic populations.10 Future research to develop similar prediction equations in other racial and ethnic groups should be considered a research priority. Furthermore, we tested only one of the various regression equations that have been developed to estimate VAT using common anthropometric, laboratory, and demographic variables.11 However, only a few of these regression equations have been validated in external cohorts.
In the future, apVAT may be considered as an ideal study endpoint in research settings to evaluate the efficacy of interventions to promote weight loss or exercise when measuring VAT with imaging modalities may not be cost-effective or feasible. Observational epidemiologic studies that integrate longitudinal measures are needed to define the minimally clinically important change in apVAT that is correlated with a meaningful change in biomarker concentrations or in the predicted risk of developing an important health event (eg type 2 diabetes or cardiovascular event). This additional data would offer clinicians and researchers a benchmark to define success of future therapeutic interventions. apVAT may also prove to be a useful screening tool to identify patients who may be most likely to benefit from weight loss of other therapeutic interventions, either in clinical practice or as an eligibility criterion for enrollment into clinical research. For example, in a clinical research setting, incorporating estimates of apVAT would allow for the accrual of a study sample that is metabolically dysregulated (eg likely to have impaired fasting glucose or metabolic syndrome) and at high risk of experiencing poor health outcomes. The utility of measuring apVAT in patient populations with established chronic diseases, such as those with cancer, pulmonary disease, and chronic kidney disease, is an area of active research.
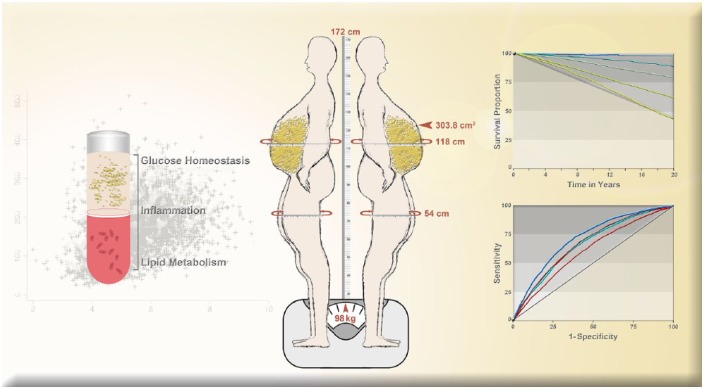
In summary, apVAT is correlated with a variety of biomarkers related to glucose homeostasis, inflammation, and lipid metabolism and explains significantly more variance in these biomarkers compared with BMI, WC, or BMI + WC.8 apVAT is associated with death from all causes, cardiovascular disease, and cancer and more accurately predicts mortality when compared with BMI, WC, and BMI + WC.9 apVAT can be used in clinical practice and in clinical nutrition and metabolism research when imaging modalities to quantify VAT are not feasible. Additional research on apVAT will continue to strengthen the importance of this measure as a tool to quantify the deleterious effects of excess adiposity on human health (Figure 1).
Figure 1.
Measurement and estimation of anthropometrically predicted visceral adipose tissue, blood-based biomarkers, and mortality outcomes.
Anthropometrically predicted visceral adipose tissue was calculated using the following validated equation (Women: 2.15 × waist circumference – 3.63 × proximal thigh circumference + 1.46 × age + 6.22 × body mass index – 92.713; Men: 6 × waist circumference – 4.41 × proximal thigh circumference + 1.19 × age – 213.65).7 The above 41-year-old male participant would have an anthropometrically predicted 303.8 cm2 of visceral adipose tissue (values rounded, center panel). Anthropometrically predicted visceral adipose tissue was correlated with and accounts for more variance in blood-based biomarkers of glucose homeostasis, inflammation, and lipid metabolism than other anthropometric measures (left panel). Moreover, anthropometrically predicted visceral adipose tissue was associated with mortality in graded dose–response fashion and more accurately predicted mortality than other anthropometric measures (right panel). Selected figures reproduced with permission.
Footnotes
Funding:Research reported in this publication was supported by the National Cancer Institute (K99-CA218603 and R25-CA203650); National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK105207); National Institute of General Medical Sciences (U54-GM104940), and National Heart, Lung, and Blood Institute (K99-HL141678).
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s Note: Justin Brown is currently affiliated with the Division of Population and Public Health Sciences at the Pennington Biomedical Research Center in Baton Rouge, Louisiana, and the Stanley S. Scott Cancer Center in New Orleans, Louisiana. Michael Harhay is currently affiliated with the Center for Clinical Epidemiology and Biostatistics in the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, Pennsylvania. Meera Harhay is currently affiliated with Division of Nephrology & Hypertension in the Department of Medicine at the Drexel University College of Medicine in Philadelphia, Pennsylvania.
Author Contributions: All authors were involved in the conception and design of this work; data analysis and interpretation; drafting of the article; critical revision of the article; and final approval of the revision to be published.
ORCID iD: Justin C Brown  https://orcid.org/0000-0001-7540-4913
https://orcid.org/0000-0001-7540-4913
References
- 1. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN. 2014;38:940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 3. Seidell JC, Bakker C, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution—a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51:953–957. [DOI] [PubMed] [Google Scholar]
- 4. Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obesity. 2007;31:1552–1553. [DOI] [PubMed] [Google Scholar]
- 5. Eastwood SV, Tillin T, Wright A, et al. Estimation of CT-derived abdominal visceral and subcutaneous adipose tissue depots from anthropometry in Europeans, South Asians and African Caribbeans. PLoS ONE. 2013;8:e75085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neamat-Allah J, Wald D, Hüsing A, et al. Validation of anthropometric indices of adiposity against whole-body magnetic resonance imaging—a study within the German European Prospective Investigation into Cancer and Nutrition (EPIC) Cohorts. PLoS ONE. 2014;9:e91586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samouda H, Dutour A, Chaumoitre K, Panuel M, Dutour O, Dadoun F. VAT = TAAT – SAAT: innovative anthropometric model to predict visceral adipose tissue without resort to CT-Scan or DXA. Obesity. 2013;21:E41–E50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown JC, Harhay MO, Harhay MN. Anthropometrically predicted visceral adipose tissue and blood-based biomarkers: a cross-sectional analysis. Eur J Nutr. 2018;57:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown JC, Harhay MO, Harhay MN. Anthropometrically-predicted visceral adipose tissue and mortality among men and women in the third national health and nutrition examination survey (NHANES III) [published online ahead of print July 17, 2016]. Am J Human Biol. doi: 10.1002/ajhb.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Camhi SM, Bray GA, Bouchard C, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring). 2011;19:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scafoglieri A, Clarys JP, Cattrysse E, Bautmans I. Use of anthropometry for the prediction of regional body tissue distribution in adults: benefits and limitations in clinical practice. Aging Dis. 2014;5:373–393. [DOI] [PMC free article] [PubMed] [Google Scholar]