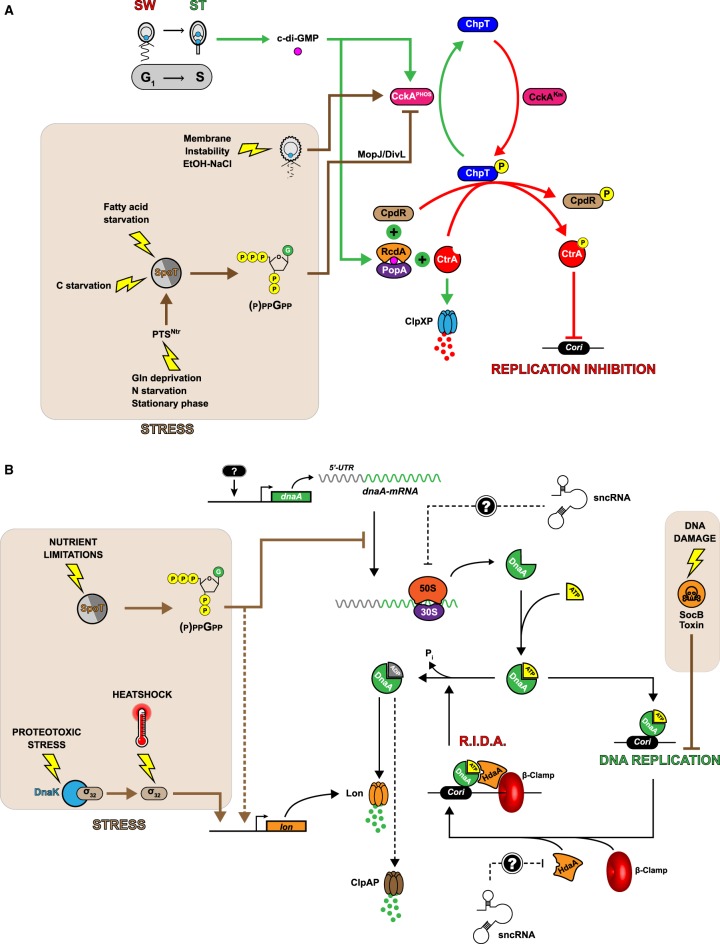
Figure 3. Graphical representation of the regulatory circuits controlling CtrA and DnaA activity in Caulobacter cells cultivated in optimal (no background color) or stressful (brown background color) growth conditions.
(A) Model for the regulatory network controlling the levels and the activity of CtrA. During the swarmer-to-stalked cell transition, the activity of CckA switches from a kinase (CckAKIN; red arrows) to a phosphatase (CckAPHOS; green arrows), reducing the levels of ChpT∼P. In turn, CtrA∼P can no more accumulate and gets actively degraded by a CpdR- and PopA/RcdA-dependent ClpXP proteolysis complex, triggering the initiation of DNA replication by DnaA-ATP. When nutrient levels become too limiting, the kinase activity of CckA is probably stimulated in a (p)ppGpp- and MopJ-dependent manner to delay the G1-to-S phase transition. In contrast, membrane stresses appear to promote the phosphatase activity of CckA and a reduction in CtrA∼P levels, maybe to delay cell division without inhibiting chromosome replication. (B) Model of the regulatory network controlling the levels and the activity of DnaA. Newly synthesized DnaA associates with ATP, which can then initiate DNA replication once CtrA∼P levels are low enough. Immediately after replication has started active DnaA-ATP is converted into inactive DnaA-ADP by the β-clamp binding HdaA protein. This conversion also appears to promote the degradation of DnaA by the Lon protease. This Regulated Inactivation and proteolysis of DnaA (RIDA) process ensures that DNA replication cannot re-initiate before the next cell cycle. Another β-clamp binding protein named SocB can inhibit the elongation of DNA replication in response to DNA damage. When Caulobacter is exposed to stresses such as nutrient limitations or proteotoxic stresses, DnaA levels drop due to an inhibition of dnaA translation and/or to a stimulation of DnaA proteolysis.