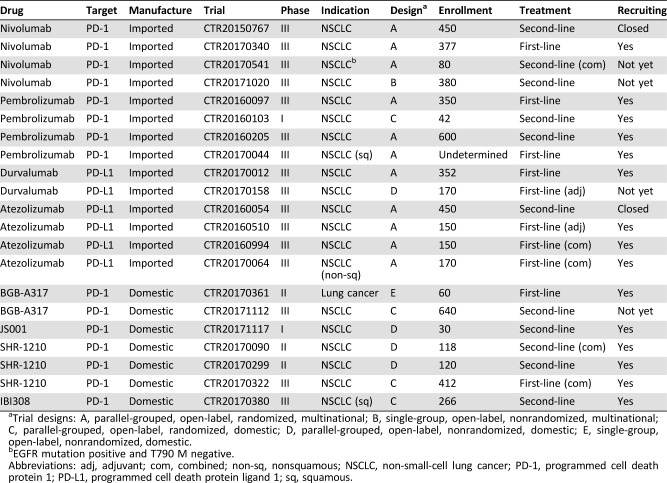
Table 3. Summary of the registered clinical trials of PD‐1/PD‐L1 blockade therapy for lung cancer in China as of October 2017.
Trial designs: A, parallel‐grouped, open‐label, randomized, multinational; B, single‐group, open‐label, nonrandomized, multinational; C, parallel‐grouped, open‐label, randomized, domestic; D, parallel‐grouped, open‐label, nonrandomized, domestic; E, single‐group, open‐label, nonrandomized, domestic.
EGFR mutation positive and T790 M negative.
Abbreviations: adj, adjuvant; com, combined; non‐sq, nonsquamous; NSCLC, non‐small‐cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death protein ligand 1; sq, squamous.