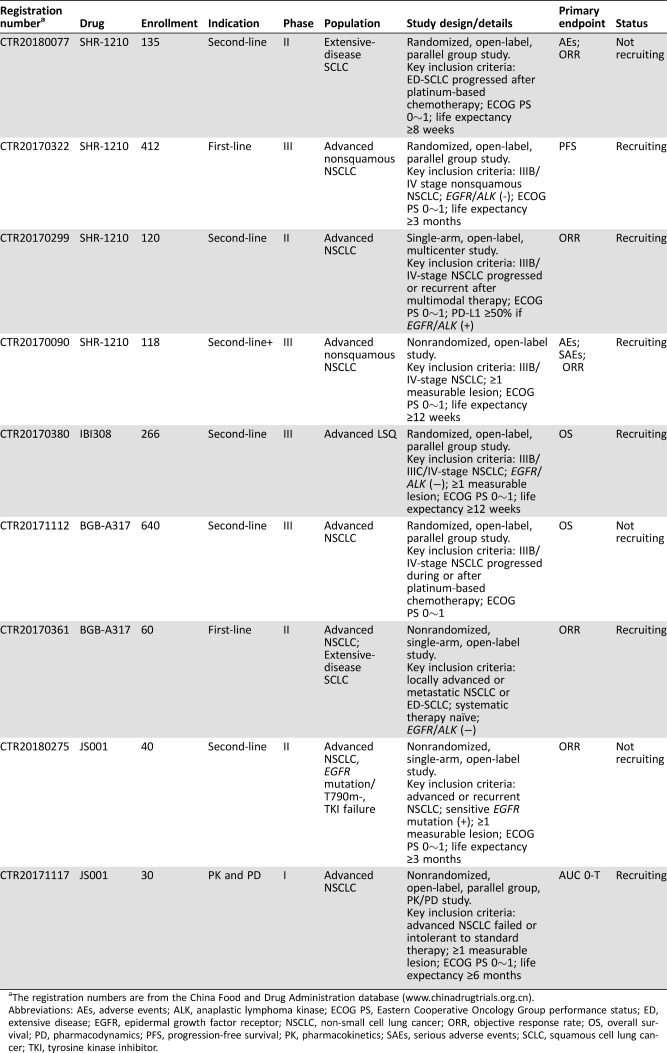
Table 4. Ongoing clinical trials with domestic programmed cell death protein 1 or programmed death‐ligand 1 inhibitors on advanced lung cancer (until April 25, 2018).
The registration numbers are from the China Food and Drug Administration database (www.chinadrugtrials.org.cn).
Abbreviations: AEs, adverse events; ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; ED, extensive disease; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; ORR, objective response rate; OS, overall survival; PD, pharmacodynamics; PFS, progression‐free survival; PK, pharmacokinetics; SAEs, serious adverse events; SCLC, squamous cell lung cancer; TKI, tyrosine kinase inhibitor.