Table 1. Results for TAm catalysed reactions of 18a–26a using phenylethanamine 5, (S)-MBA 7 and IPA 27 as amine donors for pQR2189, pQR2191, pQR2208 and Cv-TAm a , b , c .
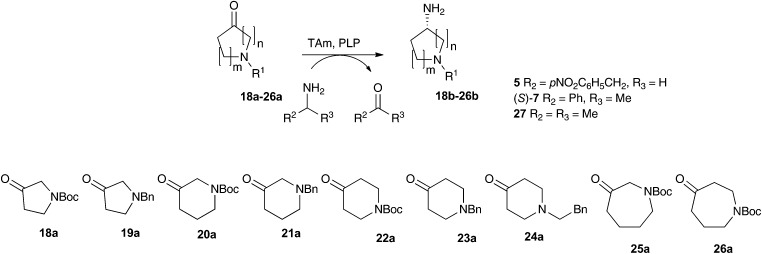
| |||||||||||
| pQR2189 |
pQR2191 |
pQR2208 |
Cv-TAm |
||||||||
| 5 | MBA (S)-7 | IPA 27 | 5 | MBA (S)-7 | IPA 27 | 5 | MBA (S)-7 | IPA 27 | 5 | MBA (S)-7 | |
| 18a |

|
43% | 72% |

|
8% | 25% |

|
45% | 67% |

|
30% |
| 19a |

|
23% | 20% |

|
5% | 4% |

|
9% | 10% |

|
6% |
| 20a |

|
30% | 60% |

|
11% | 19% |

|
30% | 55% |

|
27% |
| 21a |

|
4% | 2% |

|
68% d | 1% |

|
2% | 1% |

|
0% |
| 22a |

|
41% | Quant. e |

|
39% | Quant. e |

|
25% | 51% |

|
27% |
| 23a |

|
30% | 75% |

|
11% | 46% |

|
19% | 30% |

|
34% |
| 24a |

|
31% | Quant. e |

|
36% | Quant. e |

|
39% | 70% |

|
37% |
| 25a |

|
35% | 75% f |

|
3% | 13% f |

|
12% | 32% f |

|
n.d. g |
| 26a |

|
13% | n.d. g |

|
7% | n.d. g |

|
3% | n.d. g |

|
n.d. g |
aReaction conditions: Amine donor 5 (25 mM), amino acceptor (10 mM), PLP (0.5 mM), KPi buffer pH 7.5 (100 mM) and clarified cell extract (0.4 mg mL–1), 30 °C, 400 rpm, 18 h. Water was used as a negative control (not shown), red precipitate is indicative of activity. Assay was performed in duplicate.
bReaction conditions: Amine donor (S)-7 (25 mM), amino acceptor (5 mM), PLP (1 mM), KPi buffer pH 7.5 (100 mM) and clarified cell extract (0.4 mg mL–1), 30 °C, 400 rpm, 18 h. Acetophenone was detected at 254 nm by HPLC and used to determine percentage conversions. Water was used as a negative control, and any background levels of acetophenone production were subtracted from the reactions. All reactions were performed in triplicate, and standard deviations were <7%.
cReaction conditions: Amine donor 27 (100 mM), amino acceptor (10 mM), PLP (1 mM), KPi buffer pH 7.5 (100 mM) and clarified cell extract (0.4 mg mL–1), 35 °C, 400 rpm, 18 h. Yields were determined using HPLC against product standards. All reactions were performed in triplicate, and standard deviations were <5%.
dBy acetophenone production, product detection confirmed negligible amounts of 21b.
eQuantitative conversion.
fYields were determined by 25a depletion, but product presence was confirmed against a product standard.
gNot determined.
