Abstract
Challenges in maintaining high effectiveness of classic vector control in urban areas has renewed the interest in indoor residual spraying (IRS) as a promising approach for Aedes-borne disease prevention. While IRS has many benefits, application time and intrusive indoor applications make its scalability in urban areas difficult. Modifying IRS to account for Ae. aegypti resting behavior, named targeted IRS (TIRS, spraying walls below 1.5 m and under furniture) can reduce application time; however, an untested assumption is that modifications to IRS will not negatively impact entomological efficacy. We conducted a comparative experimental study evaluating the residual efficacy of classically-applied IRS (as developed for malaria control) compared to two TIRS application methods using a carbamate insecticide against a pyrethroid-resistant, field-derived Ae. aegypti strain. We performed our study within a novel experimental house setting (n = 9 houses) located in Merida (Mexico), with similar layouts and standardized contents. Classic IRS application (insecticide applied to full walls and under furniture) was compared to: a) TIRS: insecticide applied to walls below 1.5 m and under furniture, and b) Resting Site TIRS (RS-TIRS): insecticide applied only under furniture. Mosquito mortality was measured eight times post-application (out to six months post-application) by releasing 100 Ae. aegypti females /house and collecting live and dead individuals after 24 hrs exposure. Compared to Classic IRS, TIRS and RS-TIRS took less time to apply (31% and 82% reduction, respectively) and used less insecticide (38% and 85% reduction, respectively). Mortality of pyrethroid-resistant Ae. aegypti did not significantly differ among the three IRS application methods up to two months post application, and did not significantly differ between Classic IRS and TIRS up to four months post application. These data illustrate that optimizing IRS to more efficiently target Ae. aegypti can both reduce application time and insecticide volume with no apparent reduction in entomological efficacy.
Author summary
Vector control is the primary strategy for managing Aedes aegypti and reducing transmission of Aedes-borne diseases; however, the indoor resting behavior of Ae. aegypti and the evolution of insecticide resistance reduces the effectiveness of many vector control tactics. Indoor residual spraying (IRS) is effective against Ae. aegypti, but lengthy application time makes IRS difficult to scale within urban environments. We compared the application and entomological efficacy of Classic IRS against two novel Aedes-targeting IRS application methods (Targeted IRS [TIRS]- insecticide applied to walls below 1.5 m and under furniture and Resting Site TIRS [RS-TIRS]- insecticide applied only under furniture) within experimental houses using a carbamate insecticide. Both TIRS and RS-TIRS took less time to apply and used less insecticide compared to Classic IRS. Mortality of pyrethroid-resistant Ae. aegypti did not differ among treatments out to two months post-application, and there was no difference in mortality between Classic IRS and TIRS out to four months post-application. These data provide evidence that IRS application methods can be improved to take less time and insecticide yet not lose entomological efficacy, making TIRS more scalable within urban environments. However, larger field studies with epidemiologic endpoints are needed to further assess the efficacy of these modified TIRS techniques.
Introduction
Vector control is the principal approach for managing Aedes aegypti and reducing transmission of Aedes-borne diseases (ABD; e.g., dengue, chikungunya, Zika). Implementation of vector control targeting ABDs has primarily been in response to reports of virus transmission, using methods such as truck-mounted ultra-low volume spraying (ULV)/thermal fogging, source reduction and larviciding [1, 2]. Recent assessments of the public health value of these reactive interventions, triggered by the need to contain Zika transmission and prevent the devastating congenital malformations attributed to infection of pregnant woman, has highlighted the dearth of data supporting the role of vector control tactics in preventing ABDs [3–5]. Multiple factors challenge the efficacy and coverage of existing vector control tactics, including rapid urbanization leading to widespread Ae. aegypti distribution [6], the occurrence of cryptic larval habitats [7, 8], the rapid rise of insecticide resistance [9] and the multiplicity of virus transmission locations generated by fine-scale human mobility patterns [10, 11]. Given these challenges, management of Ae. aegypti requires highly effective, innovative approaches that can be implemented across epidemiological settings and within integrated vector management strategies [4].
Adult Ae. aegypti in urban settings typically rest indoors, where they feed frequently and almost exclusively on human blood [12–14]. This endophilic and anthropophilic behavior partially explains why outdoor space spraying (e.g., truck-mounted ultra-low volume spraying) has very limited efficacy against Ae. aegypti and ABD transmission [15]. Vector control methods that deliver insecticides indoors are more promising because they can exert a direct impact on resting adult mosquitoes [5]. The principal methods of applying insecticides indoors are indoor space spraying (ISS; application of insecticides with a droplet size of < 50 μm that kill adult vectors upon contact [5]) and indoor residual spraying (IRS; the application of aqueous formulations of insecticides with longer term residual efficacy on the walls and ceilings of houses that kill the adult vectors landing on these surfaces [16]). In terms of application and performance, ISS and IRS are very different. Indoor space spraying can be deployed rapidly, particularly during epidemics, because it can be applied quickly (< 10 min), but ISS can require up to three application cycles to achieve maximum efficacy and has a short-lived insecticidal effect, as it only targets flying mosquitoes making contact with the transient insecticidal cloud. Indoor residual spraying can provide longer-term protection after a single application; however, application time can be lengthy if all furniture and belongings need to be removed from the spray area. Despite field evidence pointing to significant epidemiological impacts of IRS in preventing dengue [5, 10, 17], and recent modeling work forecasting significant long-term reductions in disease burden after its implementation [18], the perceived labor-intensive nature of IRS (in comparison to ISS) and issues of community acceptance [19] have hindered its adoption for urban vector control targeting Ae. aegypti.
To overcome the time-consuming aspects of IRS and account for Ae. aegypti-specific behaviors, several modifications to the ‘classic’ IRS strategy intended to control vectors of malaria or Chagas disease (i.e., full house spraying, movement of furniture and treatment of all walls and ceiling) have been proposed. In Cairns, Australia, IRS is performed targeting Ae. aegypti resting sites, and insecticide is applied to exposed low walls (below 1.5 m), under furniture, inside closets and on any dark and moist surface where Ae. aegypti may be found resting [10]. This modified IRS was implemented in Cairns after the detection of local dengue transmission and dramatically reduced IRS application time and resulted in the successful containment of multiple outbreaks [10, 17, 20].
One of the untested assumptions of the modifications introduced to the classically-applied IRS is that there is no negative impact on entomological efficacy. Using a novel experimental house setting, we conducted a comparative study to evaluate the residual efficacy of classically-applied IRS against two novel IRS application methods using a non-pyrethroid insecticide against a locally-derived, pyrethroid-resistant strain of Ae. aegypti. For each IRS application method, the application time and volume of insecticide used were measured. Entomological impact over time was compared among the IRS application methods. We hypothesized that the two novel IRS application methods would provide similar levels of entomological efficacy as classically-applied IRS, but would be applied faster and use less insecticide. Furthermore, we hypothesized that the efficacy of a non-pyrethroid insecticide, specifically a carbamate insecticide (bendiocarb), would be similar between the two novel IRS application methods and classically-applied IRS.
Methods
Experimental design
Within a replicated system of nine experimental houses, we tested the residual efficacy of three IRS application methods on free flying, field-derived Ae. aegypti. The experimental houses were located in Caucel, a neighborhood at the periphery of the subtropical city of Mérida, México, and were rented long-term by the Universidad Autónoma de Yucatán (UADY) after explaining the purpose and extent of the study to the owners. Mérida is the capital of the state of Yucatán, has a population of roughly one million and experiences a rainy season from May through October. Dengue is endemic and transmission occurs throughout the year, although peak transmission occurs between July and November and corresponds with the rainy season [18, 21, 22]. Average dengue sero-prevalence rate in the population is 73.6% [23]. Since 2016, Chikungunya and Zika viruses also circulate within Merida, impacting the public health system and vector control operations [22]. Local management tactics for Ae. aegypti include ISS with either pyrethroids (e.g., deltamethrin) or organophosphates (e.g., malathion) and ULV with organophosphate insecticides (e.g., chlorpyrifos and malathion) [24]. Resistance to pyrethroids (both type I and type II) occurs in local Ae. aegypti populations, however these populations are still presently susceptible to carbamates [24–26].
Distance between experimental houses ranged from 0.3 to 2 km. The houses were similar in floor plan and design; all were concrete, single-story and had one or two living rooms, two bedrooms, one bathroom and one kitchen (Fig 1). Houses were on average 57.8 ± 2.8 m2 (mean ± SEM) and uniformly had walls 2.5 m in height. Construction characteristics were that of subsidized middle to low-income housing in Mérida, typical of areas with high ABD transmission [22].
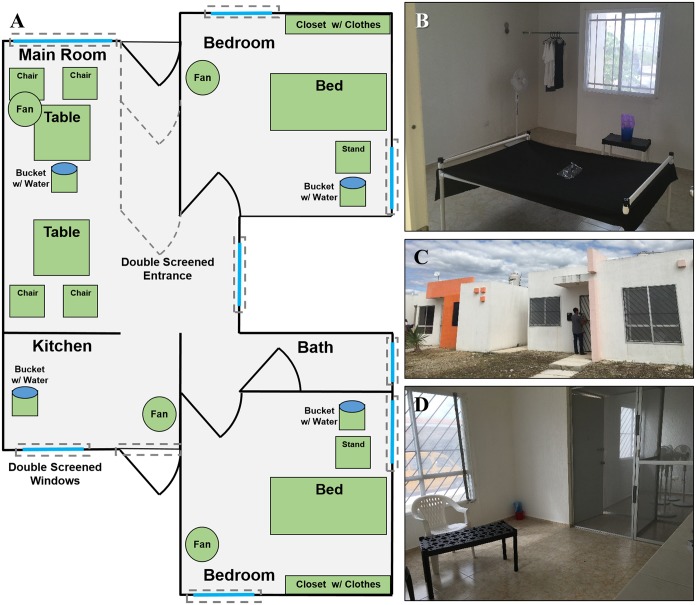
Fig 1. Layout of experimental houses.
(A) General layout, (B) setup of bedrooms, (C) exterior entrance, and (D) living room and double-screened entrance of experimental houses.
To prevent any mosquitoes used in the experiments from escaping from the houses, all windows and doors were screened on both the outside and inside of each house before the study began. Additionally, a double screened-door vestibule was built into the main entrance of each house to allow personnel to enter and exit while preventing mosquitoes from escaping (Fig 1). Sinks, drains and toilets were also sealed with window screening. Existing furniture within houses was removed, and where furniture could not be removed (e.g., built-in kitchen or closet cabinets) it was sealed with window screening. Houses were then refurnished with standardized furniture and clothing that represented typical elements found within houses (Fig 1). Furniture within in the living room (or split between two living rooms) included two black plastic tables and four plastic chairs. Within each bedroom was a bed made out of PVC tubing and black cloth, a black plastic night stand and six articles of clothing (3 black and 3 white) hung within the closet. Additionally, four plastic buckets (1 L) were half filled with water and a dark cloth and placed throughout each house to provide moisture into the environment and reduce mosquito mortality due to desiccation. Ant baits (Antex Gel, Allister de México) were placed next to each door or any other location where ants were observed to enter the experimental houses. The house layout was carefully designed to mirror elements and surface materials found in regular homes, but making sure that they were standardized in a way that allowed replication and comparability between replicates.
Insecticide application
Insecticide was applied within experimental houses on 3 July 2017. A manual compression sprayer (Hudson 93793 X-Pert) fitted with flat nozzles and a flow control valve (model CFV.R11/16SYV.ST, CFValue, Gate LLC) was used to spray houses at a flow rate of 550 mL / min. Bendiocarb (Ficam 80% WP, Bayer CropScience; 125 g sachet / 7.5 L water), a carbamate insecticide, was applied at a dosage of 0.375 g active ingredient / m2 as recommended by the WHO [16]. Bendiocarb was used because of the known susceptibility of local Ae. aegypti populations that were resistant to synthetic pyrethroids [24]. Additionally, a previous RCT in Mérida found high community acceptance of bendiocarb, with no reported adverse reactions, when it had been applied within homes [24]. The same individual applied insecticide for each of the nine experimental houses.
Houses were randomly assigned to one of three different IRS application methods: 1) Classic IRS- insecticide applied to walls and under furniture (n = 3 houses), 2) Targeted IRS (TIRS)- insecticide applied to walls below 1.5 m and under furniture (n = 3 houses) or 3) Resting Site TIRS (RS-TIRS)- insecticide only applied under furniture (n = 3 houses). Furniture was not removed from experimental houses during the insecticide application and insecticide was not applied to clothing or the plastic buckets with water. Duration of application was measured for each house, starting when the applicator entered the house and ending when the applicator exited. To estimate the volume of insecticide applied within each house, the insecticide within the sprayer was measured using a graduated cylinder before and after each application.
Mosquito strain
To test the residual efficacy of each IRS application method, a total of 100 Ae. aegypti females were released within each experimental house. The strain used (San Lorenzo strain) was locally derived, had a high level of resistance to pyrethroids and full susceptibility to carbamates [24, 26]. The San Lorenzo strain was reared and maintained at the insectaries of the Unidad Colaborativa para Bioensayos Entomológicos, UADY, Mérida, México. Mosquitoes released into houses were three to seven days old from the F4 generation, before release had only been provided sugar solution and were non-bloodfed.
Post-insecticide application, mosquitoes were released into the experimental houses eight times over a six month period; 1) +1 day, 2) +14 days, 3) +1 month, 4) +2 months, 5) +3 months, 6) +4 months, 7) +5 months and 8) +6 months. To facilitate mosquito recovery, all experimental houses were vacuumed and swept clean of any debris on the floor one day prior to mosquito release. After 24 hrs exposure, a team of four field technicians entered each house and searched for live mosquitoes using a Prokopack aspirator [27] and searched by hand for dead mosquitoes. Searching for Ae. aegypti ceased when either 100 mosquitoes were collected or > 20 minutes elapsed after the last mosquito was collected (circa 30–40 min / house). Natural mortality within experimental houses was measured by placing three unsprayed control cups (250 mL) within each house, with each cup containing 10 San Lorenzo strain females. Control cups were placed within experimental houses simultaneously during the main release of mosquitoes during the +4, +5 and +6 months post-application evaluations. After searching for released Ae. aegypti ceased, the number of live and dead Ae. aegypti within control cups were counted.
Statistical analyses
For each sampling period, mortality was calculated per house by dividing the number of dead individuals by the number of individuals released. Missing individuals were assumed to be dead. Mortality was compared between IRS application methods using mixed-model analysis of variance (ANOVA) in R 3.2 statistical software (https://www.r-project.org/). Sampling date, IRS application method, and their interaction were classified as fixed effects and experimental house was classified as a random effect. When significant differences were detected, pairwise comparisons were made using LSMEAN package and alpha levels were adjusted for multiple comparisons using the Tukey correction. Additionally, regression analysis was used to assess the relationship between application time and volume of insecticide applied among the three IRS application methods.
Ethics statement
This was an experimental study, and because mosquitoes were released into uninhabited houses rented on long-term contracts, we did not require an Institutional Review Board.
Results
Insecticide application
Compared to Classic IRS, TIRS reduced application time on average by 5.8 min / house (31.3% reduction), whereas RS-TIRS reduced application time on average by 15.2 min / house (82.0% reduction) (Table 1). Similarly, compared to Classic IRS, TIRS used on average 2.02 L / house less insecticide (37.9% reduction), while RS-TIRS saved on average 4.53 L / house (84.8% reduction) (Table 1). Compared to TIRS, RS-TIRS reduced both application time by 9.40 min / house (73.8% reduction) and insecticide volume by 2.50 L / house (75.5% reduction) (Table 1). Reductions in both application time and insecticide volume were significantly linear (F = 140.1; df = 1, 7; P < 0.0001), indicating consistent insecticide application among IRS application methods.
Table 1. Application time and volume of insecticide applied within experimental houses for the three IRS application modes.
| Application Method | Total Areaa Range (Mean % Treated) |
Application Time (min) |
Volume Applied (L) |
|---|---|---|---|
| Classic IRS | 137–141 m2 (100 ± 0%)b | 18.6 ± 3.1b | 5.34 ± 0.58b |
| Targeted IRS | 137–173 m2 (65.8 ± 0.6%) | 12.7 ± 1.1 | 3.32 ± 0.18 |
| Resting Site Targeted IRS | 160–171 m2 (5.9 ± 0.1%) | 3.3 ± 0.4 | 0.81 ± 0.11 |
a Total area = sum area of walls plus the area of the furniture within the experimental house.
b Mean ± Standard error of the mean
Mosquito recovery and mortality
A total of 7,200 Ae. aegypti females were released within the experimental houses throughout the trial. Mosquito recovery averaged 96.9 ± 0.82% (Mean ± SEM; n = 72 releases). Based on pilot data, we attribute high recovery to pre-cleaning the floors of experimental houses the day before mosquitoes were released and to effective management of ants using baits.
Mortality within control cups average 4.4 ± 1.3%, 1.5 ± 0.7% and 5.0 ± 1.7% (Mean ± SEM) for evaluations from +4, +5 and +6 months post-application, respectively, indicating high Ae. aegypti survival within the experimental house environments.
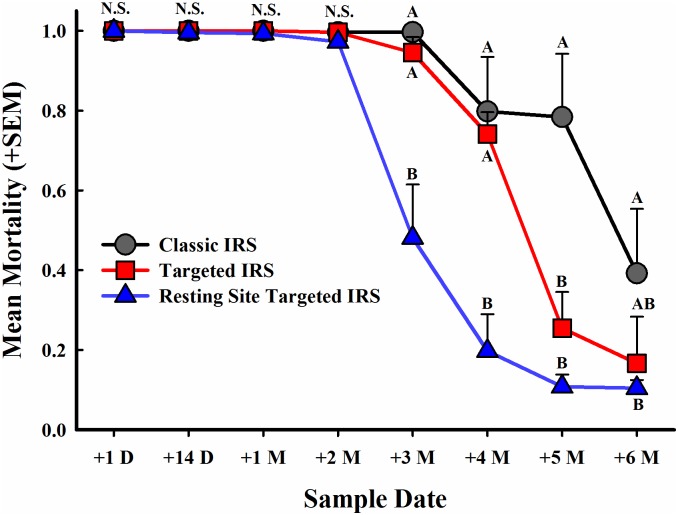
There was a significant interaction between IRS application method and sampling time post application (F = 6.3; df = 14, 42; P < 0.0001) (Fig 2). Almost complete mortality of all released mosquitoes was observed up to two months post-application (ranging from 97.3 to 100%); there were no significant differences in mortality among the three IRS treatments within the first 4 sampling periods. At three months post-application, mortality of Ae. aegypti dropped significantly in houses treated with RS-TIRS (from 97.3% at +2 months to 48.1% at +3 months) compared to Classic IRS and TIRS houses, where mortality remained high (99.7% and 94.5%, respectively). At four months post-application, mortality of Ae. aegypti from Classic IRS and TIRS treated houses dropped to 79.8% and 74.2%, respectively, but were both significantly greater compared to mortality of Ae. aegypti from RS-TIRS houses, which dropped to 19.7%. Mortality in experimental houses with Classic IRS remained high five months post-application (78.4%) and was significantly greater compared to both TIRS (25.5%) and RS-TIRS (10.8%), which did not differ from each other. Efficacy of all three treatments was greatly reduced six months post-application (one month beyond the expected residual duration of bendiocarb). Mortality in Classic IRS treated houses was reduced to 39.2%, yet was significantly greater compared to RS-TIRS (10.4%), although neither treatment differed significantly from TIRS (16.6%) (Fig 2).
Fig 2. Mortality of pyrethroid-resistant Ae. aegypti by IRS application method using bendiocarb over time.
Symbols denote sample means and error bars are the standard error of the mean. Letters denote significant differences among IRS application methods within sample date.
Discussion
We compared the residual efficacy of Classic IRS against two novel IRS application methods, TIRS and RS-TIRS, in experimental houses, and hypothesized that the two novel IRS application methods would be as efficacious as Classic IRS. Furthermore, we hypothesized that the efficacy of a non-pyrethroid insecticide, bendiocarb, would be similar among the two novel IRS application methods and Classic IRS. Although both TIRS and RS-TIRS took less time to apply and used less insecticide compared to Classis IRS (Table 1), these data support our hypotheses, as pyrethroid-resistant Ae. aegypti mortality did not differ among the three IRS application methods up to two months post-application and did not differ between Classic IRS and TIRS up to four months post-application (Fig 2).
Using bioassays within experimental houses that closely simulate typical living conditions, this study provides important information that can help improve the mode of IRS application and cost-effectiveness within the urban context of ABD transmission. Improvements in IRS efficiency and application are key for increasing scalability and adoption of this management tactic [28]. Recent and rapid scaling-up of IRS for malaria control illustrate the potential public health benefits of this approach [29], but also point to the difficulties of reaching and sustaining high coverage levels due to IRS’s labor-intensive nature [30]. If IRS were to be widely adopted for urban Ae. aegypti management, lessons from IRS scale-up for malaria vector control should be taken into consideration to better frame the operational conditions and approaches for intervention delivery.
Field observational studies from Central and South America have found that Ae. aegypti primarily rest indoors and below 1.5 m, particularly on or near dark places such as behind or under furniture, under beds, on clothing and on lower parts of walls [13, 27, 31]. This low-resting behavior has also been observed in experimental hut studies using an Ae. aegypti strain from Thailand [32]. Modifying IRS to account for key Ae. aegypti resting behaviors resulted in important reductions in application time and insecticide volume (Table 1) without sacrificing entomological efficacy for two to four months post application (Fig 2). The fact that we detected high mortality with no statistical difference between Classic IRS and TIRS methods show that Ae. aegypti are not avoiding treated locations by shifting resting behaviors above 1.5 m. Additionally, RS-TIRS was applied only to common resting sites (beds, chairs and other furniture) and resulted in to up to 2 months of full protection, providing further evidence of the remarkable preference of Ae. aegypti for specific resting locations.
Duration of protection differed between TIRS and RS-TIRS applications. Although RS-TIRS could be completed on average in 3.3 min / house (Table 1), the protection provided (using > 80% mortality as a threshold) by this approach lasted two months, or half the duration of Classic IRS or TIRS (Fig 2). One of the challenges of RS-TIRS when applied in real households (which would likely be more cluttered and full of personal items than our experimental houses) is that it may entail the treatment of personal belongings that are preferentially used by Ae. aegypti as resting sites (e.g., suitcases, clothes, etc.). Applying insecticide to personal belongings could potentially lead to community disapproval of the methodology, as well as potentially result in unanticipated exposure to insecticides [19]. As such, while there are significant reductions in application time and insecticide volume, performing RS-TIRS may be more challenging than performing TIRS. Given that TIRS provides longer-term protection (up to 4 months) compared to RS-TIRS, we see the former as a methodology highly suitable for implementation within the context of urban Ae. aegypti management.
A randomized controlled trial evaluating the entomological impact of Classic IRS using bendiocarb against pyrethroid-resistant populations of Ae. aegypti in Mérida, México, demonstrated a 65–75% reduction in adult Ae. aegypti abundance in treatment clusters, compared to controls, up to three months post-application [24]. Furthermore, the application time of Classic IRS from this trial averaged approximately 30 min / house [24]. Our experimental study demonstrated that an application of TIRS required roughly 12 min to complete but resulted in a 4-month protection of treated houses. The residual effects observed were driven by the insecticide used (bendiocarb residuality is expected to last between 3 and 5 months), and its interaction with treated substrates (in our case, painted walls, cloth, wood and plastic). Given the recent development of new residual insecticide formulations for malaria, which extend residual duration out to 6–8 months and are effective against pyrethroid-resistant mosquitoes [33, 34], there is potential for extending residual power of TIRS beyond the 4-month mark.
Despite the higher cost of novel insecticide formulations, applying novel insecticides via TIRS would not only reduce application time but also potentially increase cost-effectiveness. Furthermore, extending residual duration can provide a longer window of protection and shift IRS application from reactive (in response to reported clinical cases, as in [10]) to pro-active (performed prior to the transmission season [18]). A recent analysis of historical dengue, chikungunya and Zika cases geocoded to the household level found a significant level of spatial overlap of the three pathogens within specific geographic units that accumulated more than half of all cases [22]. The pro-active (pre-season) deployment of high-quality interventions such as TIRS within hot-spot areas could offer additional protection to areas that consistently report high rates of ABD transmission [22, 35]. An insecticide with residual duration that lasts more than 5 months could protect a household for an entire transmission season (which lasts 5 to 6 months) using a single TIRS application. Additionally, using insecticides pro-actively should be coupled with insecticide-resistance monitoring and insecticides used for TIRS changed when resistance is first detected. Previous studies have demonstrated that fitness costs associated with pyrethroid resistance in Aedes aegypti do exist and that susceptibility can be regained in the absence of selection [36]. While the efficacy of such pro-active TIRS implementation in preventing ABD will require further evaluations with proper epidemiologic endpoints [37], the findings presented here provide clear evidence for how IRS applications could be optimized for urban Aedes management. However, larger field studies with epidemiologic endpoints are needed to further assess the efficacy of these modified TIRS techniques.
Acknowledgments
The authors would like to thank Carlos Alberto Arisqueta Chablé, Yolanda Carolina Carmona Carballo, Suemy Analí Gutiérrez Martín, Eduardo José Geded Moreno, and Ana Laura Marrufo Tamayo for their dedication and efforts. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Data Availability
Data are available at Open Science Framework (OSF): Dunbar et al Efficacy of Novel IRS Methods Targeting Pyrethroid-Resistant Aedes aegypti within Experimental Houses; URL https://osf.io/3hksx/.
Funding Statement
This project received support from Emory Global Health Institute and Marcus Foundation (project #00052002), Emory Global Health Institute Seed Grant (project) the Centers for Disease and Prevention (CDC: OADS BAA 2016-N- 17844) and Mexico’s CONACYT (Project # 000000000255141). SAR is funded by National Health and Medical Research Council Senior Research Fellowship 1044698. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Reiter P, Gubler DJ. Surveillance and control of urban dengue vectors In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorragic fever. Wallingford, UK: CAB International; 1997. [Google Scholar]
- 2.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control: World Health Organization; 2009. [PubMed] [Google Scholar]
- 3.Bowman LR, Donegan S, McCall PJ. Is Dengue Vector Control Deficient in Effectiveness or Evidence?: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10(3):e0004551 10.1371/journal.pntd.0004551 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achee NL, Gould F, Perkins TA, Reiner RC Jr., Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9(5):e0003655 10.1371/journal.pntd.0003655 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel M, Maoz D, Manrique P, Ward T, Runge-Ranzinger S, Toledo J, et al. Community effectiveness of indoor spraying as a dengue vector control method: A systematic review. PLoS Negl Trop Dis. 2017;11(8):e0005837 10.1371/journal.pntd.0005837 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubler DJ. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Tropical medicine and health. 2011;39(4 Suppl):3–11. 10.2149/tmh.2011-S05 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008;22(1):62–9. Epub 2008/04/03. 10.1111/j.1365-2915.2008.00720.x . [DOI] [PubMed] [Google Scholar]
- 8.Manrique-Saide P, Arisqueta-Chable C, Geded-Moreno E, Herrera-Bojorquez J, Valentin UC, Chable-Santos J, et al. An assessment of the importance of subsurface catch basins for Aedes aegypti adult production during the dry season in a neighborhood of Merida, Mexico. J Am Mosq Control Assoc. 2013;29(2):164–7. Epub 2013/08/08. 10.2987/12-6320R.1 . [DOI] [PubMed] [Google Scholar]
- 9.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11(7):e0005625 10.1371/journal.pntd.0005625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazquez-Prokopec GM, Montgomery BL, Horne P, Clennon JA, Ritchie SA. Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Science advances. 2017;3(2):e1602024 10.1126/sciadv.1602024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A. 2013;110(3):994–9. Epub 2013/01/02. 10.1073/pnas.1213349110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadee DD. Resting behaviour of Aedes aegypti in Trinidad: with evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasites & vectors. 2013;6(1):255 10.1186/1756-3305-6-255 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzul-Manzanilla F, Ibarra-Lopez J, Bibiano Marin W, Martini-Jaimes A, Leyva JT, Correa-Morales F, et al. Indoor Resting Behavior of Aedes aegypti (Diptera: Culicidae) in Acapulco, Mexico. J Med Entomol. 2017;54(2):501–4. 10.1093/jme/tjw203 . [DOI] [PubMed] [Google Scholar]
- 14.Liebman KA, Stoddard ST, Reiner RC Jr., Perkins TA, Astete H, Sihuincha M, et al. Determinants of heterogeneous blood feeding patterns by Aedes aegypti in Iquitos, Peru. PLoS Negl Trop Dis. 2014;8(2):e2702 10.1371/journal.pntd.0002702 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15(5):619–31. 10.1111/j.1365-3156.2010.02489.x . [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets. Zaim/WHOPES DR, editor. Geneva: WHO; 2006. [Google Scholar]
- 17.Vazquez-Prokopec GM, Kitron U, Montgomery B, Horne P, Ritchie SA. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl Trop Dis. 2010;4(12):e920 10.1371/journal.pntd.0000920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hladish TJ, Pearson CAB, Patricia Rojas D, Gomez-Dantes H, Halloran ME, Vazquez-Prokopec GM, et al. Forecasting the effectiveness of indoor residual spraying for reducing dengue burden. PLoS Negl Trop Dis. 2018;12(6):e0006570 Epub 2018/06/26. 10.1371/journal.pntd.0006570 providing quality, unbiased scientific consulting for hire; it sells no other product or service. All authors declare that no competing interests exist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz-Soldan VA, Bauer KM, Hunter GC, Castillo-Neyra R, Arriola VD, Rivera-Lanas D, et al. To spray or not to spray? Understanding participation in an indoor residual spray campaign in Arequipa, Peru. Glob Public Health. 2018;13(1):65–82. Epub 2016/05/18. 10.1080/17441692.2016.1178317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie SA, Long S, Smith G, Pyke A, Knox TB. Entomological investigations in a focus of dengue transmission in Cairns, Queensland, Australia, by using the sticky ovitraps. J Med Entomol. 2004;41(1):1–4. Epub 2004/03/03. . [DOI] [PubMed] [Google Scholar]
- 21.Hladish TJ, Pearson CA, Chao DL, Rojas DP, Recchia GL, Gomez-Dantes H, et al. Projected Impact of Dengue Vaccination in Yucatan, Mexico. PLoS Negl Trop Dis. 2016;10(5):e0004661 Epub 2016/05/27. 10.1371/journal.pntd.0004661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisanzio D, Dzul-Manzanilla F, Gomez-Dantes H, Pavia-Ruz N, Hladish TJ, Lenhart A, et al. Spatio-temporal coherence of dengue, chikungunya and Zika outbreaks in Merida, Mexico. PLoS Negl Trop Dis. 2018;12(3):e0006298 10.1371/journal.pntd.0006298 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavia-Ruz N, Diana Patricia R, Salha V, Granja P, Balam-May A, Longini IM, et al. Seroprevalence of Dengue Antibodies in Three Urban Settings in Yucatan, Mexico. The American journal of tropical medicine and hygiene. 2018;98(4):1202–8. 10.4269/ajtmh.17-0382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez-Prokopec GM, Medina-Barreiro A, Che-Mendoza A, Dzul-Manzanilla F, Correa-Morales F, Guillermo-May G, et al. Deltamethrin resistance in Aedes aegypti results in treatment failure in Merida, Mexico. PLoS Negl Trop Dis. 2017;11(6):e0005656 10.1371/journal.pntd.0005656 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saavedra-Rodriguez K, Beaty M, Lozano-Fuentes S, Denham S, Garcia-Rejon J, Reyes-Solis G, et al. Local Evolution of Pyrethroid Resistance Offsets Gene Flow among Aedes aegypti Collections in Yucatan State, Mexico. American Journal of Tropical Medicine and Hygiene. 2015;92(1):201–9. 10.4269/ajtmh.14-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deming R, Manrique-Saide P, Medina Barreiro A, Cardena EU, Che-Mendoza A, Jones B, et al. Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasites & vectors. 2016;9:67 Epub 2016/02/06. 10.1186/s13071-016-1346-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46(6):1256–9. Epub 2009/12/08. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knapp J, Macdonald M, Malone D, Hamon N, Richardson JH. Disruptive technology for vector control: the Innovative Vector Control Consortium and the US Military join forces to explore transformative insecticide application technology for mosquito control programmes. Malar J. 2015;14:371 Epub 2015/09/28. 10.1186/s12936-015-0907-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimnig JE, Otieno P, Were V, Marwanga D, Abong’o D, Wiegand R, et al. The Effect of Indoor Residual Spraying on the Prevalence of Malaria Parasite Infection, Clinical Malaria and Anemia in an Area of Perennial Transmission and Moderate Coverage of Insecticide Treated Nets in Western Kenya. PLoS One. 2016;11(1):e0145282 Epub 2016/01/06. 10.1371/journal.pone.0145282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen DA, Borrill L, Patel R, Fregosi L. Reported community-level indoor residual spray coverage from two-stage cluster surveys in sub-Saharan Africa. Malaria Journal. 2017;16(1):249 10.1186/s12936-017-1893-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perich M, Davila G, Turner A, Garcia A, Nelson M. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. Journal of medical entomology. 2000;37(4):541–6. [DOI] [PubMed] [Google Scholar]
- 32.Tainchum K, Polsomboon S, Grieco JP, Suwonkerd W, Prabaripai A, Sungvornyothin S, et al. Comparison of Aedes aegypti (Diptera: Culicidae) resting behavior on two fabric types under consideration for insecticide treatment in a push-pull strategy. Journal of medical entomology. 2013;50(1):59–68. [DOI] [PubMed] [Google Scholar]
- 33.Uragayala S, Kamaraju R, Tiwari SN, Sreedharan S, Ghosh SK, Valecha N. Village-scale (Phase III) evaluation of the efficacy and residual activity of SumiShield((R)) 50 WG (Clothianidin 50%, w/w) for indoor spraying for the control of pyrethroid-resistant Anopheles culicifacies Giles in Karnataka state, India. Tropical medicine & international health: TM & IH. 2018. Epub 2018/03/31. 10.1111/tmi.13056 [DOI] [PubMed] [Google Scholar]
- 34.Oxborough RM, Kitau J, Jones R, Feston E, Matowo J, Mosha FW, et al. Long-lasting control of Anopheles arabiensis by a single spray application of micro-encapsulated pirimiphos-methyl (Actellic® 300 CS). Malaria Journal. 2014;13:37-. 10.1186/1475-2875-13-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hladish TJ, Pearson CAB, Patricia Rojas D, Gomez-Dantes H, Halloran ME, Vazquez-Prokopec GM, et al. Effectiveness of Indoor Residual Spraying for Reducing Dengue Transmission. (in prep). [DOI] [PMC free article] [PubMed]
- 36.Grossman MK, Uc-Puc V, Rodriguez J, Cutler DJ, Morran LT, Manrique-Saide P, et al. Restoration of pyrethroid susceptibility in a highly resistant Aedes aegypti population. Biology letters. 2018;14(6). 10.1098/rsbl.2018.0022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiner RC Jr., Achee N, Barrera R, Burkot TR, Chadee DD, Devine GJ, et al. Quantifying the Epidemiological Impact of Vector Control on Dengue. PLoS Negl Trop Dis. 2016;10(5):e0004588 10.1371/journal.pntd.0004588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at Open Science Framework (OSF): Dunbar et al Efficacy of Novel IRS Methods Targeting Pyrethroid-Resistant Aedes aegypti within Experimental Houses; URL https://osf.io/3hksx/.