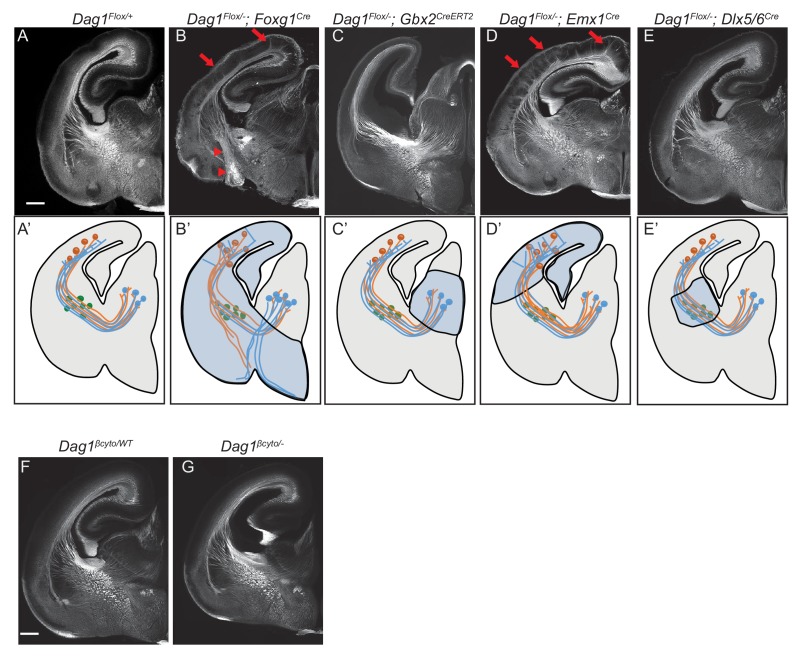
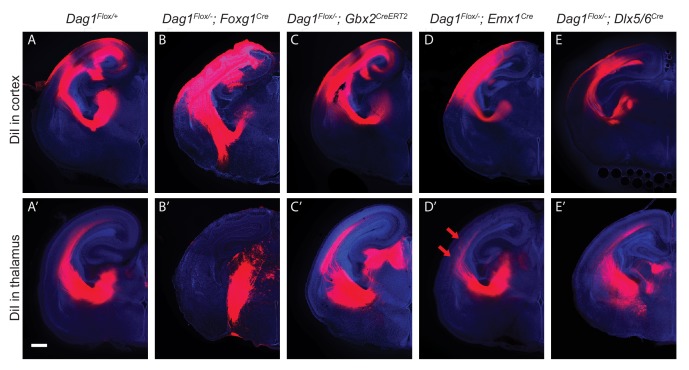
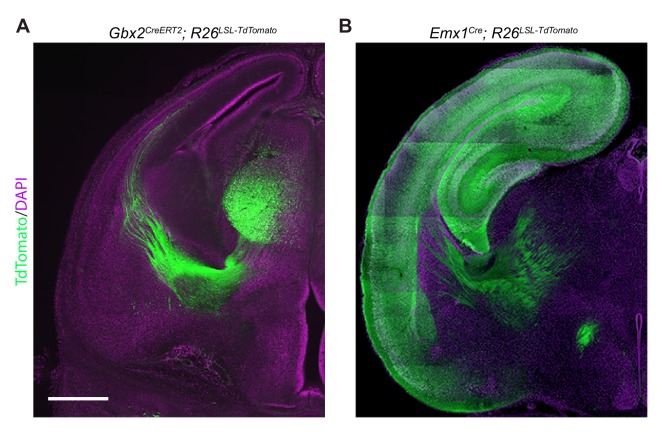
Figure 3. Dystroglycan is required in ventral telencephalon neuroepithelial cells to guide corticothalamic and thalamocortical axons.
L1 staining of P0 brain sections from Dag1F/+ controls (n = 3 animals) (A, F), Dag1F/-;Foxg1Cre (n = 4 animals) (B), Dag1F/-;Gbx2CreERT2 (n = 4 animals) (C), Dag1F/-;Emx1Cre (n = 3 animals) (D), Dag1F/-;Dlx5/6Cre (n = 3 animals) (E), and Dag1βcyto/- (n = 5 animals) (G). A’-E’ illustrate the recombination patterns in each Cre/CreERT2 line in the blue shaded area. Deletion of Dystroglycan throughout the neuroepithelium of the dorsal and ventral telencephalon in Dag1F/-;Foxg1Cre mutants (B, B’) results in abnormal projections in the internal capsule (red arrowheads) and abnormal axonal projections into the upper layers of the cortex (red arrows). Deletion of Dystroglycan from the neuroepithelium of the dorsal telencephalon with Emx1Cre mutants (D) results in abnormal axonal projections into the upper layers of the cortex (red arrows), but normal internal capsule formation. Deletion of Dystroglycan from the thalamus with Gbx2CreERT2 (C) or ‘corridor’ cells with Dlx5/6Cre (E) did not affect axon guidance. Deletion of the intracellular domain of Dystroglycan in Dag1βcyto/- mutants (G) did not affect formation of the internal capsule compared to control littermates (F). A-G Scale bar = 500 μm.