Abstract
Enhanced Recovery after Surgery (ERAS) protocols have been demonstrated to improve hospital length of stay and outcomes in patients undergoing colorectal surgery. This article presents the specific components of an ERAS protocol implemented at the authors' institution. In particular, details of both surgical and anesthetic ERAS pathways are provided with explanation of all aspects of preoperative, perioperative, and postoperative care. Evidence supporting inclusion of various aspects within the ERAS protocol is briefly reviewed. The ERAS protocol described has significantly benefitted postoperative outcomes in colorectal patients and can be employed at other institutions wishing to develop an ERAS pathway for colorectal patients. A checklist is provided for clinicians to easily reference and facilitate implementation of a standardized protocol.
Keywords: enhanced recovery, colorectal, protocol
An ERAS Protocol for Elective Colorectal Procedures
Enhanced Recovery after Surgery (ERAS) pathways have been shown to reduce postoperative complications and hospital length of stay in patients undergoing elective colorectal surgery. 1 2 An ERAS protocol for colectomy patients was first introduced at our institution, Massachusetts General Hospital, in 2014, and has since been expanded to all colorectal operations. Introduction of the ERAS pathway required very close collaboration with various providers and nurses taking part in the patient's journey through various phases of care. The implementation began after five town hall meetings that included representatives from surgery, anesthesia, preoperative testing, intraoperative nursing, and postanesthesia recovery room and floor nursing. Following a careful discussion of various aspects of the pathway with these various members of the team, a small pilot of 30 patients was first performed. Each case was followed carefully throughout all phases of care and a debrief meeting was held to determine which steps of the pathway required refinement or fine-tuning. After the first 30 cases, an audit was conducted to make sure the surgical outcomes did not deteriorate. We were pleased to find that the audit showed a 1 day reduction in patient length of stay and a 60% reduction in postoperative complications, including surgical infections. Readmission rates remained unchanged. Armed with this reassuring data, the ERAS pathway was rolled out to all colorectal resections. A few modifications were added prior to the official roll out to include surgical site infection (SSI) reduction steps. Since then, we have followed the protocol in over 500 patients undergoing colorectal resection. The patients on the protocol continue to be closely followed postoperatively, and the data suggest continued reduction in length of stay with excellent outcomes with regard to rates of infections, anastomotic leaks, and renal and cardiac complications (these data are currently under review for publication).
This article summarizes the Massachusetts General Hospital ERAS pathway as it is currently being practiced, with some supporting evidence and useful implementation advice.
Preoperative Phase
Patient Education
Central to the success of the ERAS pathway at our institution has been active patient education across multiple levels of patient care. Surgeons engage all patients in a discussion focused on the operation, as well as both preoperative optimization and postoperative recovery at the initial outpatient office visit. This discussion sets expectations for patient care with the shared goal of early recovery and discharge. All patients receive perioperative ERAS instructions at the initial outpatient visit with a detailed outline of all components of pre- and postoperative care, as well as key patient objectives for preoperative preparation, including nutrition, hydration, bowel preparation, and infection control. After the initial office visit, a dedicated patient education nurse practitioner (NP) is notified of all patients enrolled in the protocol. The role of the patient education NP is to counsel patients through the preoperative process, answering all questions and alleviating anxiety. Intensive preoperative education appears to have a positive impact on hospital length of stay, especially in a population of patients enrolled in an ERAS pathway. 3 4 Preliminary data from our institution suggest that patient education NP may independently decrease hospital length of stay for patients within an ERAS protocol.
Nutrition
Patients are instructed to hydrate and eat carbohydrate-rich meals 2 days prior to surgery to ensure that patients enter the perioperative period in an anabolic state optimal for wound healing and recovery of preoperative functional status. In addition, we encourage patients to remain well hydrated while undergoing their preoperative mechanical and antibiotic bowel preparation (see more details later). With this goal in mind, we recommend all patients drink two bottles (24 oz.) of Gatorade before midnight on the day before surgery. Patients are then NPO at midnight (for 7:30 a.m. cases), except for a preoperative nutritional supplemental drink given 3 hours before induction of anesthesia, which needs to be fully ingested no later than 2 hours prior to the induction of anesthesia. Patients scheduled for afternoon surgery are allowed to drink clear liquids until 3 hours prior to surgery. When the patient reaches the 3-hour prior to surgery mark, they are asked to drink a carbohydrate drink containing at least 45 g of complex carbohydrates in at least 400 mL of fluid (e.g., 24 oz of ClearFast). At our institution, this drink is provided to the patient free of charge. Alternatively, up to 20 oz of Gatorade may be used as an alternative. This practice is based on randomized control trial data demonstrating preoperative carbohydrate loading leads to a significantly reduced postoperative hospital stay in patients undergoing colorectal surgery when compared with patients who undergo usual preoperative fasting protocols. 5 Additionally, preoperative carbohydrate-rich drinks have been shown to reduce preoperative thirst, hunger, and anxiety, as well as postoperative insulin resistance. 6 7
Infection Control
To optimize infection control, patients are instructed to shower or bathe with liquid chlorhexidine soap 2 days prior to and on the morning of surgery. Patients are additionally given instructions for mechanical and oral antibiotic preparation for all colon resections. A wide variety of mechanical preparation regimens to be taken on the day prior to surgery are acceptable and include (1) two to four Dulcolax pills at 2 p.m. followed by one bottle of Miralax in 64 oz of clear liquid taken from 3 to 5 p.m.; (2) 4 L of GoLytely preparation from noon to 4 p.m.; (3) two to four Dulcolax pills at 2 p.m. followed by one bottle of Mg citrate at 3 p.m. A combination of at least two antibiotics (Neomycin 1,000 mg/erythromycin 500 mg or neomycin 1,000 mg/metronidazole 500 mg) should be given 1 hour after completion of any of the aforementioned mechanical bowel preps for three doses. We emphasize to the patient that the antibiotics need to be taken after the ingestion of the bowel prep has been completed ( Fig. 1 ).
Fig. 1.
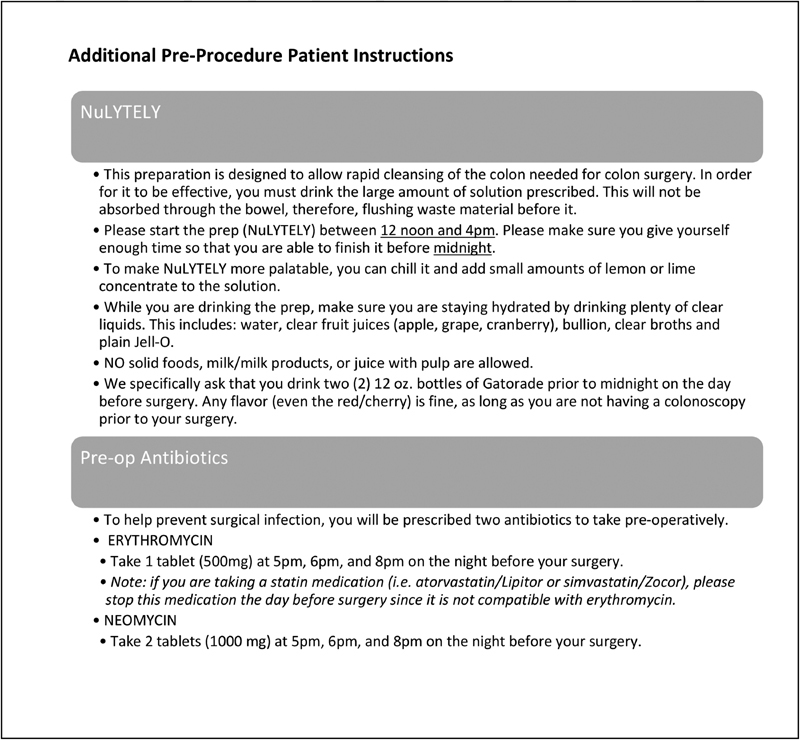
Mechanical and oral antibiotic bowel preparation instructions.
Analgesia
Preemptive analgesia is administered to patients on arrival to the preoperative care unit, approximately 2 hours prior to the scheduled surgery. Typically, patients are asked to take acetaminophen 1,000 mg and gabapentin 600 mg by mouth with a sip of clear liquid. The dose of gabapentin is adjusted for elderly patients. Alternative agents include celecoxib 400 mg by mouth and ketorolac, depending on surgeon preference and patient renal function. Our institution does not routinely administer preoperative anxiolytic medications due to unpredictable effects on postoperative recovery and sedation.
Perioperative Phase
Anesthesia
An anesthetic plan that will facilitate recovery and return of gastrointestinal motility is critical to the enhanced recovery pathway. The primary goals of anesthesia have been described in a “trimodal approach”—limitation of the stress response to surgery, fluid balance, and analgesia. 8 Short-acting anesthetic agents are generally preferred for induction and continuous anesthesia (propofol, cisatracurium, fentanyl), allowing for attenuation of sympathetic stimulation and for postoperative recovery to begin as early as possible in the postoperative care unit. Intraoperative maintenance of normal physiologic parameters is additionally achieved by temperature monitoring via esophageal probe every 5 minutes to ensure normothermia, and hourly glucose monitoring to prevent hyperglycemia. Regional anesthetic blocks are often utilized to minimize intraoperative anesthetic requirements, as well as to reduce postoperative opiate use. Epidural catheters are placed preoperatively for most patients undergoing open abdominal surgery. Ideally, the epidural is placed at the T7–T9 level for right-side resections and T9–T0 level for left-side and rectal resections. Bupivacaine 0.2% at 3 mL/hour is used and often reduces hypotension. Epidural anesthesia has been shown to impede recovery after laparoscopic colorectal resections 9 and is therefore avoided in such cases. Instead, most patients undergoing laparoscopic surgery receive a transverse abdominis plane (TAP) block. Postoperative TAP blocks facilitate shorter length of stay in ERAS protocols in an efficient and cost-effective manner 10 and have been shown to be noninferior to epidural analgesia for laparoscopic colorectal surgery. 11 TAP blocks may be placed preoperatively; however, postoperative placement is preferred to prevent case delay. Placement is assisted by ultrasound guidance, and the preferred agent is ropivacaine 0.5%. If epidural anesthesia is not used, particularly in open cases, additional anesthetic options commonly utilized include ketamine (both for induction and maintenance) and ketorolac, again with the goal of minimizing postoperative narcotic use.
Antiemetics
Postoperative nausea and vomiting is a common impediment to early recovery. As previously described, anesthetic agents are selected to optimize return of gastrointestinal motility and decrease postoperative ileus. Furthermore, all patients receive intraoperative antiemetics for prophylaxis of postoperative nausea and vomiting. Common agents administered before the end of the case include Decadron 4 to 6 mg IV (early in the case), Zofran 4 mg IV (30 minutes before the end of the case), and Haldol 1 mg IV (used at variable time points depending on anesthesiologist preference).
Fluid Management
Perioperative fluids are managed judiciously in the ERAS protocol, as liberal use of fluids has been demonstrated to delay return of normal gastrointestinal function 12 and may increase postoperative complications. 13 A strict protocol has been used to dictate fluid management at our institution. First, patients are not administered fluids in the preoperative holding area. If patients are hypotensive at induction, a bolus of 5 to 7 mL/kg of crystalloid is administered. Maintenance crystalloid infusion of up to 2 mL/kg/hour for laparoscopic cases and 3 mL/kg/hour for open cases is recommended, though this is not always easy to follow, and some members of the anesthesia team utilize additional monitoring at their discretion. For most cases, simple physiologic monitoring is achieved with a bladder catheter, blood pressure cuff, and pulse oximetry. Generally, urine output of 0.2 mL/kg/hour is considered acceptable if other physiologic parameters suggest euvolemia. Intraoperative hypotension is approached first by determining the etiology. If a patient is demonstrating a distributive physiology and is not hypovolemic, vasopressor medications are used. If patients are hypovolemic, patients may be given up to two boluses of 250 mL of colloid. If patients remain hypotensive, the anesthesiologist may proceed to a goal-directed fluid therapy protocol with device-based monitoring. At present, the esophageal Doppler is the only evidence-based option 14 and is used to ensure adequate left ventricular stroke volume before further titration of vasopressors. This being said, there are at least two other devices that have recently entered the market that seem to achieve similar outcomes.
Infection Control
Due to the nature of most colorectal procedures, the operative field is often contaminated and wound infection becomes a significant consideration. As is standard in all colorectal surgery, patients receive a preoperative antibiotic regimen within 60 minutes of incision. Chloraprep is the preferred agent for skin preparation; however, Duraprep is also used depending on surgeon preference. Careful attention is given to handling of dirty surgical equipment. When an enterotomy or colotomy is made, an additional tray or mayo stand is brought to the field. All instruments used during this portion of the operation are taken from and placed on this surface until the bowel is closed. If another clean instrument is needed from the sterile table, another sterile instrument must be used to retrieve the instrument to prevent contamination from dirty gloves. If drapes are contaminated, a sterile towel is placed over the contaminated area. After the bowel is closed, all instruments including suction tips and electrocautery are passed placed on the tray and passed off the field. All surgeons and assistants change gloves and gowns if contaminated, before proceeding to the next portion of the operation. To further minimize wound infection, wound protectors are routinely used in open cases and at the time of bowel division in laparoscopic cases. The wound protector is covered in towels at the time of enterotomy/colotomy. Once the dirty portion of the procedure is finished, the wound protector is removed without contamination of subcutaneous tissue. If the wound protector is contaminated during the case, it should be removed and replaced.
Drains/Tubes
The routine use of intra-abdominal drains and nasogastric tubes should be avoided in patients in an ERAS protocol. Randomized trials have shown that patients who do not receive nasogastric tubes in the immediate postoperative period have no difference in nausea, vomiting, time to return of bowel function, or increased length of stay. 15 16 17 Similarly, evidence suggests that intra-abdominal drainage does not improve postoperative outcomes and is associated with drain-related complications, and thus should be avoided. 18 19 20 If Foley catheters are used intraoperatively to monitor urine output, they should be removed in the operating room at the end of the case, if possible.
Postoperative Care
Enhanced Recovery after Surgery Colorectal Order Template
Members of both surgery and anesthesia departments have access to a standard set of ERAS postoperative order sets that includes all components of the ERAS pathway described here. These order sets help ensure adherence to the specific protocol for all designated ERAS patients across a variety of services performing colectomies within the hospital (colorectal surgery, acute care surgery, surgical oncology, gynecologic oncology, transplant surgery). All faculty and house staff who are involved in the care of ERAS patients received a laminated copy of the “checklist” ( Fig. 2 ) for easy reference in the postoperative period. Of note, postoperative order templates include specific orders for both the postanesthesia care unit (PACU) and the inpatient surgical floor.
Fig. 2.
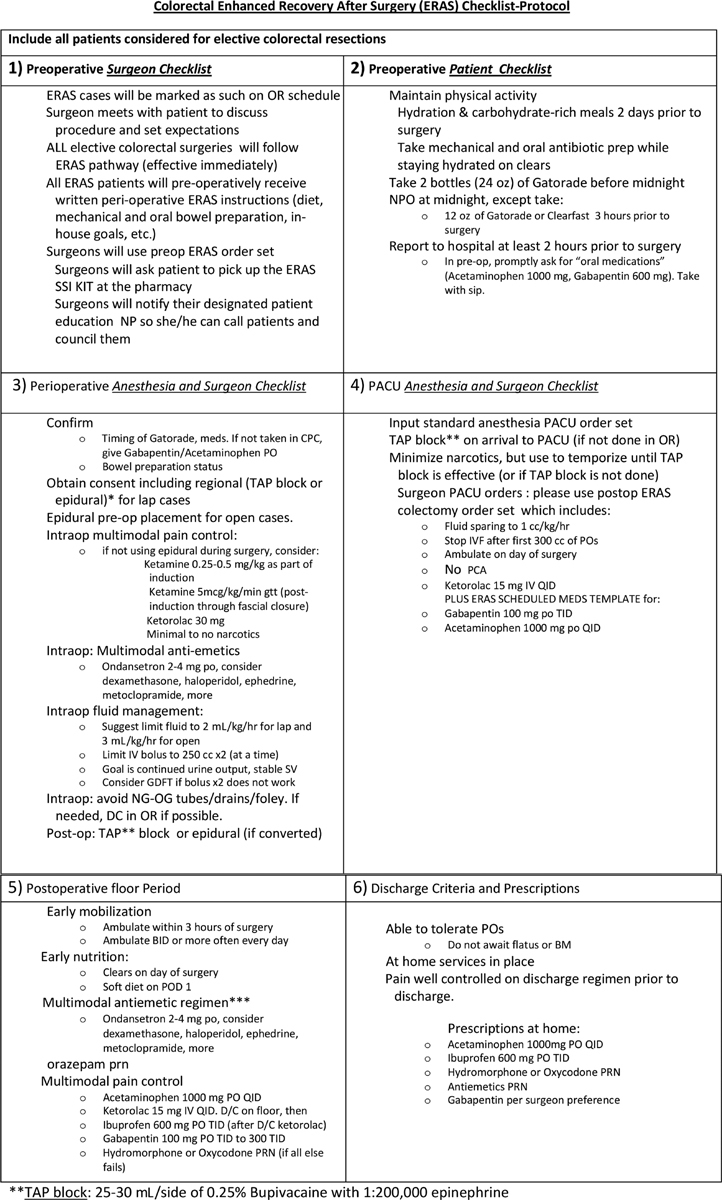
( A ) and ( B ). Enhanced Recovery after Surgery (ERAS) protocol checklist: This checklist provides an important clinical reference tool for physicians implementing an ERAS protocol, and contains all important aspects including preoperative care, perioperative anesthetic and surgical care, and postoperative management guidelines.
Early Mobilization
Early mobilization is strongly encouraged in the ERAS pathway. All patients who have undergone either laparoscopic or open surgery are instructed to ambulate at least from the bed to chair within 3 hours of arriving to the PACU. Upon arrival to the floor, patients are asked to ambulate at least once on postoperative day 0. On postoperative day 1, patients ambulate in the hallway at least three times daily. For patients having undergone abdominoperineal resection (APR), sitting is not permitted until postoperative day 2, at which point patients may sit on a soft surface (pillow or cushion). The benefits of early mobilization are multifold—increased muscle strength, decrease in pulmonary complications, and earlier return of bowel function.
Analgesia/Antiemetics
A multimodal postoperative analgesic regimen is employed with the goal of allowing for early mobilization and promoting early return of gastrointestinal function. As mentioned earlier, epidural analgesia is administered for patients who have had open surgery and TAP blocks are often used for laparoscopic surgery. Randomized control trials have demonstrated that midthoracic epidural analgesia is associated with an earlier return of bowel function and tolerance of an oral diet compare with opiate-based analgesia. 21 Epidural catheters are removed by postoperative day 2, or earlier if patients are tolerating PO. Once the epidural catheter is removed, analgesia is achieved with nonsteroidal anti-inflammatory drugs (Toradol and/or ibuprofen if kidney function is acceptable), standing gabapentin, and standing Tylenol. Patients with TAP blocks are administered standing Tylenol and gabapentin from the onset. Opiates are reserved only for breakthrough pain and patient-controlled analgesia (PCAs) are used only in patients with chronic narcotic dependence.
Fluid Management
Postoperative fluid management parallels intraoperative fluid management: judicious administration of resuscitation and maintenance fluids with the goal of maintaining hemodynamic stability while preventing the adverse effects of fluid overload and edema. Crystalloids are run at 1 mL/kg/hour and discontinued postoperatively after 6 hours or 300 mL of oral intake, whichever occurs first. If hypotension occurs, up to three boluses of 250 mL of crystalloid or colloid are given for resuscitation. Specific criteria for requiring a bolus are systolic blood pressure greater than 15% below normal, mean arterial pressure less than 65, or urine output less than 0.25 mL/kg/hour. If patients continue to be unresponsive to fluids, more objective measures of fluid status are utilized in a goal-directed manner. The most commonly used measure is simple bedside echocardiography, which all house staff are taught to perform.
Nutrition
Patients are allowed to drink clear liquids immediately in the postoperative period once awake and able to safely drink. Diet is advanced as tolerated on postoperative day 1 after a clear liquid breakfast, if patients are clinically progressing. If nausea or vomiting develops, advancement is delayed until symptoms resolve. Early diet advancement has been shown to improve outcomes with earlier return of bowel function and shorter length of stay with no increase in nasogastric tube reinsertion or anastomotic complications. 22
Urinary Catheter
Urinary catheters are often placed intraoperatively for monitoring of urine output as well as bladder decompression in patients with epidural catheters; however, they are a nidus for urinary tract infection. It is standard at our institution that all urinary catheters placed in patients who are within an ERAS protocol be removed in the operating room for colonic resections and within 72 hours for rectal resections. In cases of urinary retention, replaced catheters are removed within 48 hours.
Discharge Criteria
Patients must meet a defined set of criteria prior to discharge. First, the patient must be able to tolerate an oral diet sufficient to meet nutritional needs. Importantly, patients are not required to stay as inpatients until return of flatus or bowel movements, contrary to usual practice on general surgical services. Despite this, patients are discharged home with no apparent increase in readmission for postoperative ileus. Second, postoperative pain must be well controlled on a multimodal oral pain regimen while in the hospital. This multimodal regimen includes Tylenol, ibuprofen, gabapentin, and oral oxycodone or Dilaudid, and patients are given prescriptions for a 2-week supply of all medications at discharge. Specific discharge criteria are often individualized based on patient characteristics and complexity of the operation; however, all patients must meet the aforementioned criteria.
Summary
Institution of an enhanced recovery protocol for patients undergoing colon and rectal resections requires comprehensive input and collaboration from both surgery and anesthesia teams. Importantly, protocols should follow detailed pathways for preoperative, perioperative, and postoperative phases of patient care and all aspects of care should be aligned to optimize patient nutrition, fluid status, gastrointestinal function, and pain control. Protocols inclusive of these principles have been demonstrated to reduce inpatient length of stay and improve surgical outcomes. The logistics of rolling out an ERAS protocol within a department can be facilitated by the utilization of standardized order sets, open communication between surgeons, anesthesiologists, house staff, and nursing; as well as distribution of a simplified checklist to all providers caring for patients within the protocol.
Footnotes
Conflict of Interest None declared.
References
- 1.Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(06):1531–1541. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 2.Varadhan K K, Neal K R, Dejong C H, Fearon K C, Ljungqvist O, Lobo D N. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29(04):434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Younis J, Salerno G, Fanto D, Hadjipavlou M, Chellar D, Trickett J P. Focused preoperative patient stoma education, prior to ileostomy formation after anterior resection, contributes to a reduction in delayed discharge within the enhanced recovery programme. Int J Colorectal Dis. 2012;27(01):43–47. doi: 10.1007/s00384-011-1252-2. [DOI] [PubMed] [Google Scholar]
- 4.Phatak U R, Li L T, Karanjawala B, Chang G J, Kao L S. Systematic review of educational interventions for ostomates. Dis Colon Rectum. 2014;57(04):529–537. doi: 10.1097/DCR.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 5.Noblett S E, Watson D S, Huong H, Davison B, Hainsworth P J, Horgan A F. Pre-operative oral carbohydrate loading in colorectal surgery: a randomized controlled trial. Colorectal Dis. 2006;8(07):563–569. doi: 10.1111/j.1463-1318.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 6.Hausel J, Nygren J, Lagerkranser M et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(05):1344–1350. doi: 10.1097/00000539-200111000-00063. [DOI] [PubMed] [Google Scholar]
- 7.Nygren J, Soop M, Thorell A, Efendic S, Nair K S, Ljungqvist O. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17(02):65–71. doi: 10.1016/s0261-5614(98)80307-5. [DOI] [PubMed] [Google Scholar]
- 8.Levy B F, Scott M J, Fawcett W J, Day A, Rockall T A. Optimizing patient outcomes in laparoscopic surgery. Colorectal Dis. 2011;13 07:8–11. doi: 10.1111/j.1463-1318.2011.02770.x. [DOI] [PubMed] [Google Scholar]
- 9.Hübner M, Blanc C, Roulin D, Winiker M, Gander S, Demartines N. Randomized clinical trial on epidural versus patient-controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg. 2015;261(04):648–653. doi: 10.1097/SLA.0000000000000838. [DOI] [PubMed] [Google Scholar]
- 10.Keller D S, Ermlich B O, Delaney C P. Demonstrating the benefits of transversus abdominis plane blocks on patient outcomes in laparoscopic colorectal surgery: review of 200 consecutive cases. J Am Coll Surg. 2014;219(06):1143–1148. doi: 10.1016/j.jamcollsurg.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Niraj G, Kelkar A, Hart E et al. Comparison of analgesic efficacy of four-quadrant transversus abdominis plane (TAP) block and continuous posterior TAP analgesia with epidural analgesia in patients undergoing laparoscopic colorectal surgery: an open-label, randomised, non-inferiority trial. Anaesthesia. 2014;69(04):348–355. doi: 10.1111/anae.12546. [DOI] [PubMed] [Google Scholar]
- 12.Lobo D N, Bostock K A, Neal K R, Perkins A C, Rowlands B J, Allison S P.Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial Lancet 2002359(9320):1812–1818. [DOI] [PubMed] [Google Scholar]
- 13.Brandstrup B, Tønnesen H, Beier-Holgersen R et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(05):641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasa S, Taylor M H, Sammour T, Kahokehr A A, Hill A G. Oesophageal Doppler-guided fluid administration in colorectal surgery: critical appraisal of published clinical trials. Acta Anaesthesiol Scand. 2011;55(01):4–13. doi: 10.1111/j.1399-6576.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 15.Lei W Z, Zhao G P, Cheng Z, Li K, Zhou Z G. Gastrointestinal decompression after excision and anastomosis of lower digestive tract. World J Gastroenterol. 2004;10(13):1998–2001. doi: 10.3748/wjg.v10.i13.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feo C V, Romanini B, Sortini D et al. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. 2004;74(05):298–301. doi: 10.1111/j.1445-1433.2004.02985.x. [DOI] [PubMed] [Google Scholar]
- 17.Petrelli N J, Stulc J P, Rodriguez-Bigas M, Blumenson L. Nasogastric decompression following elective colorectal surgery: a prospective randomized study. Am Surg. 1993;59(10):632–635. [PubMed] [Google Scholar]
- 18.Merad F, Yahchouchi E, Hay J M, Fingerhut A, Laborde Y, Langlois-Zantain O; French Associations for Surgical Research.Prophylactic abdominal drainage after elective colonic resection and suprapromontory anastomosis: a multicenter study controlled by randomization Arch Surg 199813303309–314. [DOI] [PubMed] [Google Scholar]
- 19.Merad F, Hay J M, Fingerhut A et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. Surgery. 1999;125(05):529–535. [PubMed] [Google Scholar]
- 20.Denost Q, Rouanet P, Faucheron J L et al. To drain or not to drain infraperitoneal anastomosis after rectal excision for cancer: the GRECCAR 5 randomized trial. Ann Surg. 2017;265(03):474–480. doi: 10.1097/SLA.0000000000001991. [DOI] [PubMed] [Google Scholar]
- 21.Taqi A, Hong X, Mistraletti G, Stein B, Charlebois P, Carli F. Thoracic epidural analgesia facilitates the restoration of bowel function and dietary intake in patients undergoing laparoscopic colon resection using a traditional, nonaccelerated, perioperative care program. Surg Endosc. 2007;21(02):247–252. doi: 10.1007/s00464-006-0069-5. [DOI] [PubMed] [Google Scholar]
- 22.Dag A, Colak T, Turkmenoglu O, Gundogdu R, Aydin S. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (Sao Paulo) 2011;66(12):2001–2005. doi: 10.1590/S1807-59322011001200001. [DOI] [PMC free article] [PubMed] [Google Scholar]


