Abstract
In the present study, follicle-sinus complexes (FSCs) were harvested from the muzzle skin of 123 dogs with suspected canine rabies, and the sensitivity and specificity of FSC analysis were compared with those of brain tissue immunohistochemistry analysis. In the FSCs, viral antigen was detected from Merkel cells. Sensitivity was 97.3%, specificity was 100%, and the coefficient κ was 0.88. These results reconfirm that FSCs are very useful for the postmortem diagnosis of canine rabies, and suggest that 5 FSCs can yield results that are almost equivalent to those derived from brain tissue analysis in rabid dogs.
Keywords: Follicle-sinus complex, FSC, post-mortem diagnosis, rabies
Rabies is an incurable affliction and a serious zoonotic disease. More than 150 countries and territories currently report rabies infections in humans, and there are >55,000 annual deaths, of which approximately 34,500 occur in Asian countries [15]. In the Philippines, domestic dogs are the primary reservoirs of rabies and more than 98% of human rabies-associated deaths are due to dog bites [6].
The most widely used test for rabies diagnosis is the direct fluorescent antibody test (dFAT), which is recommended by both the World Organization for Animal Health (OIE) [10] and the World Health Organization (WHO) [14]. The sensitivity of the dFAT is 100% when the brain samples are fresh, and definitive diagnosis can be completed within a few hours. However, when brain samples start to decompose due to warm temperatures, sensitivity declines and the method becomes unsuitable [1, 7]. The sampling and testing of dog brain tissue is also laborious, is associated with a high risk of virus exposure, and requires expensive equipment including a fluorescence microscope. Therefore, the identification and characterization of alternative types of specimens for the postmortem diagnosis of canine rabies via low-cost, simple tests that are associated with a low risk of viral exposure are required in rabies-endemic countries.
In animals, the tactile hair, known as the follicle-sinus complex (FSC), is a specialized touch organ that is abundant in the muzzle skin. Each tactile hair is equipped with more than 2,000 sensory nerve endings [8]. Therefore, tactile hairs may act as alternative postmortem diagnostic material. In addition, obtaining FSCs from the muzzle skin of dogs that have died is easy and practical, and does not require any specialized and expensive equipment. We recently reported that in dogs, Merkel cells in the FSCs of the muzzle skin are targets of rabies virus infection and replication [12, 13]. However, the number of FSCs required for a reliable histopathological diagnosis was not evaluated. In the present study, the sensitivity and specificity of rabies virus antigen detection using 5 FSCs per dog was investigated in a sample of 123 dogs suspected of rabies infection, and compared with the analysis of brain tissues via immunohistochemistry (IHC) and the dFAT. The present study is a continuation of our previous published study and provides more information on the post-mortem diagnostic utility of FSCs in the muzzle skin of rabid dogs.
Muzzle skin and brain samples (hippocampus and medulla oblongata) were obtained from the cadavers of 123 dogs suspected of having rabies that were submitted to the Research Institute for Tropical Medicine (RITM) in the Philippines for postmortem rabies testing. Ninety dogs had been found dead, and 33 had been euthanized. The 123 dogs (62 male, 45 female, and 16 of unknown sex) ranged in age from 1 month to >15 years, and 13 dogs were of unknown age. Five of the 123 dogs had a known history of rabies vaccination, 53 reportedly had no history of rabies vaccination, and no information on rabies vaccination status was available for the remaining 65. Primary clinical symptoms of canine rabies infection such as unprovoked aggressiveness, incessant biting of inanimate objects, aimless running, and excessive salivation had reportedly been observed in 86 of the 123 dogs, and no information was available for the remaining dogs. Small transverse sections (2–3 mm in thickness) of the hippocampus and medulla oblongata of each rabies-suspected dog were excised, and a slide was touched against the cut surfaces. The slides were then fixed in cold acetone overnight, then air-dried at room temperature (RT). A 450-µl preparation of fluorescein isothiocyanate-conjugated anti-rabies monoclonal antibody (Fujirebio, Malvern, PA, U.S.A.) was added to the slides, and they were incubated for 30 min at 37°C in a high-humidity chamber. They were then rinsed 20–25 times in phosphate-buffered saline twice, followed by a distilled water wash. Small amounts of mounting medium (20% glycerol in Tris-buffered saline, pH 9.0) were placed on the slides, then coverslips were applied and they were examined under a fluorescence microscope (80i, Nikon, Tokyo, Japan).
The hippocampus, medulla oblongata, and muzzle skin samples of the 123 rabies-suspected dogs were fixed in 10% neutral-buffered formalin at RT for ≥72 hr. In a preliminary experiment, first we determined the number of rows of FSCs in the muzzle skin, after shaving, and cut it in a horizontal direction. Grossly, four rows of the FSCs were confirmed in the muzzle skin (Fig. 1); the second row was the longest and it contained the largest number of FSCs (6 to 7 FSCs), and the size of FSCs was larger towards the caudal side than the nose side (Fig. 2). To decide a suitable number for the statistical study, 3, 4, and 5 FSCs in the muzzle skin were randomly derived from 10 rabid dogs and each was tested for the distribution of viral antigens. We found no differences in the localization of the viral antigen in the rows in the muzzle skin; in contrast, the viral antigen-positive rate increased in proportion to the number of FSCs. The viral antigen-positive rate of 1 or more of the 3 FSCs was 80%, 1 or more of the 4 FSCs was 90%, 1 or more of the 5 FSCs was 100%, respectively (Table 1). Therefore, in this study, 5 FSCs (either the left or right) per dog were excised from the second row in the muzzle skin using surgical scissors, tweezers, and a trimming knife, and were cut in a longitudinal direction as described in a previous report [12]. The brain tissue samples and FSCs were embedded in paraffin, sectioned at a thickness of 3 µm, and mounted. The sections were stained with hematoxylin and eosin (HE), and serial sections were subjected to IHC for detecting rabies viral antigen. For the detection of rabies viral antigen in brain tissues and FSCs, sections were stained using the polymer method with rabbit polyclonal rabies anti-phosphoprotein antibody (anti-P) as previously described [4, 12, 14]. Briefly, tissue sections were treated with 0.25% trypsin at RT for 30 min for anti-P antibody. To remove endogenous peroxidase activity, tissue sections were immersed in 0.3% H2O2 in methanol. To block nonspecific reactions, the sections were treated with 10% normal goat serum (Nichirei Biosciences, Tokyo, Japan). Sections were incubated overnight with the primary antibody at 4°C in a humidified chamber (1:1,200). Histofine® Simple StainTM MAX PO (rabbit) (Nichirei Biosciences) was used to detect the primary antibody. The antibody was visualized using 3-3′-diaminobenzidine (Nichirei Biosciences). Lastly, the sections were counterstained with hematoxylin.
Fig. 1.
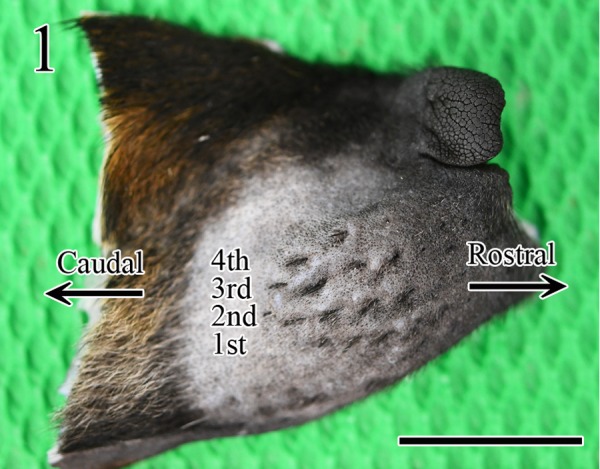
Gross image of an FSC in the muzzle skin, after shaving. Four rows of FSCs were observed. Bar=1 cm.
Fig. 2.
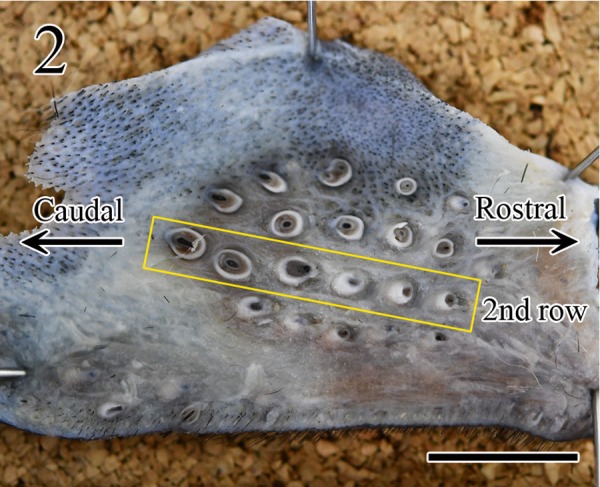
Gross image of the horizontal cut surface of an FSC. The second row was the longest and contained 6 FSCs, and the size of FSCs was larger towards the caudal than rostral direction. Bar=0.5 mm.
Table 1. Result of the viral antigen-positive rate in 3 FSCs, 4 FSCs and 5 FSCs in the muzzle skins of 10 rabid dogs.
| Number of FSCs | Number of viral antigen-positive dogs in FSCs/number of tested dogs |
Viral antigen-positive rate (%) |
|---|---|---|
| 3 FSCs | 8/10 | 80 |
| 4 FSCs | 9/10 | 90 |
| 5 FSCs | 10/10 | 100 |
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) with 95% confidence interval (CI), and coefficient κ were calculated for the correct identification of rabies. The positive and negative results of dFAT and IHC of brain tissues were considered true-positive and true-negative indicators of rabies because the dFAT test is the gold standard for rabies diagnosis [10, 15]. Hypothesis testing for the difference in population proportions was used to compare the sensitivity of FSC examination in the found dead and euthanized cases (significance set at P<0.05; two-tailed z-test).
Hippocampus and medulla oblongata specimens of 110 of the 123 suspected rabid dogs were antigen-positive for dFAT and IHC, respectively and the remaining 13 dogs were negative by both the dFAT and IHC (Table 2). Histopathological findings including Negri bodies were not observed in any of the FSCs, and viral antigen was concentrated in a part of the outer root sheath at the level of the ring-wulst in the FSCs (Fig. 3). The morphology and localization of positive cells were consistent with Merkel cells [12, 13].
Table 2. Results of the IHC examination of brain (hippocampus and medulla oblongata) and 5 FSCs of 123 rabies suspected dogs.
| Number and specimens | IHC positive | IHC negative |
|---|---|---|
| 123 (brain) | 110 | 13 |
| 123 (FSCs) | 107 | 16 |
Fig. 3.
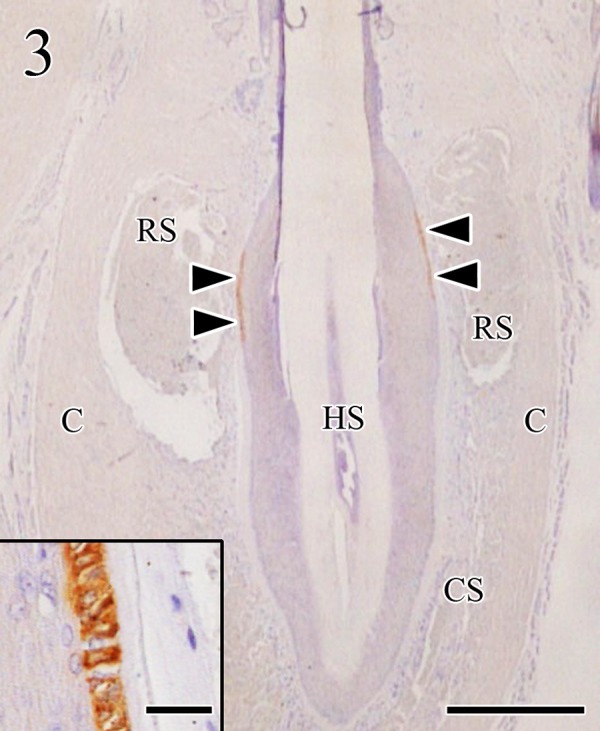
Viral antigens were concentrated in a part of the outer root sheath of the FSC (arrowheads) at the level of the ring sinus. Virus antigens were localized in the cytoplasm of Merkel cells (insert). C: capsule, CS: cavernous sinus, HS: hair shaft, RS: ring sinus. Immunohistochemistry. Bar=500 µm (insert, bar=25 µm).
The Merkel cells in the FSCs of 107 of 123 rabies-suspected dogs were true-positive for viral antigen by IHC, whereas 13 were true-negative and 3 were false-negative. In the 3 false-negative dogs, 2 dogs were found dead and 1 dog was euthanized. Unfortunately, the detailed clinical information for these 3 dogs was not obtained. The sensitivity, specificity, PPV, NPV with 95% CI, and coefficient κ of FSCs in all samples were 97.3% (95% CI 91.8–99.5), 100% (95% CI 96.4–100.0), 100% (95% CI 96.4–100.0), 81.3% (95% CI 72.6–88.1), and 0.88, respectively (Table 3). In the found-dead specimens, the Merkel cells in the FSCs of 78 of 90 rabies-suspected dogs were true-positive for viral antigen by IHC, whereas 10 were true-negative and 2 were false-negative. The sensitivity, specificity, PPV, NPV with 95% CI, and coefficient κ of FSCs in all samples were 97.5% (95% CI 92.3–99.6), 100% (95% CI 96.4–100.0), 100% (95% CI 96.4–100.0), 83.3% (95% CI 75.1–90.3), and 0.9, respectively. In the euthanized specimens, the Merkel cells in the FSCs of 29 of 33 rabies-suspected dogs were true-positive for viral antigen by IHC, whereas 3 were true-negative and 1 was false-negative. The sensitivity, specificity, PPV, NPV with 95% CI, and coefficient κ of FSCs in all samples were 96.7% (95% CI 91.2–99.2), 100% (95% CI 96.4–100.0), 100% (95% CI 96.4–100.0), 75.0% (95% CI 65.6–82.9), and 0.84, respectively. There was no statistically significant difference in sensitivity between the found-dead and euthanized dogs (Table 4).
Table 3. Comparison of sensitivity, specificity, PPV, NPV with 95% CI, and coefficient κ for detecting viral antigen of 110 FSCs specimens using IHC method (95% CI in parentheses).
| Specimens | Sensitivity | Specificity | PPV | NPV | Coefficient κ |
|---|---|---|---|---|---|
| FSCs | 97.3 (91.8–99.5) | 100.0 (96.4–100.0) | 100.0 (96.4–100.0) | 81.3 (72.6–88.1) | 0.88 |
Table 4. Comparison of sensitivity, specificity, PPV, NPV with 95% CI, and coefficient κ for detecting viral antigen of 110 FSCs specimens by manner of death, using IHC method (95% CI in parentheses).
| Manner of death | Sensitivitya) | Specificity | PPV | NPV | Coefficient κ |
|---|---|---|---|---|---|
| Found dead | 97.5x (92.3–99.6) | 100.0 (96.4–100.0) | 100.0 (96.4–100.0) | 83.3 (75.1–90.3) | 0.9 |
| Euthanasia | 96.7x (91.2–99.2) | 100.0 (96.4–100.0) | 100.0 (96.4–100.0) | 75.0 (65.6–82.9) | 0.84 |
a) Values superscripted with the different letters differed significantly in a comparison based on two-tailed z-test (significance set at <0.05) in sensitivity
Skin biopsies are generally used for antemortem and postmortem diagnosis of rabies in humans [3]. The virus antigen is detected in the peripheral nerves surrounding the hair follicles, and their number increases with the progression of the clinical course of infection [2, 3]. Previously, immunofluorescence-based routine laboratory tests for the antemortem diagnosis of rabies in human skin biopsy specimens indicated that a minimum of 20 sections was required to observe the hair follicles [5]. In addition, the skin biopsy sample should contain at least 10 hair follicles [9]. In the present study, the sensitivity and specificity of tests conducted using 5 FSCs were >95%, indicating a high true-positive rate and a low false-negative rate. The coefficient κ was 0.88, indicating that the standard method using brain tissue and the FSC method exhibited almost complete agreement [11]. In both found-dead and euthanized dogs, sensitivity was 95% and specificity was 100%, and respective coefficient κ values were 0.9 and 0.84. These results reconfirm that FSCs are very useful for the postmortem diagnosis of canine rabies. Notably however, in 3/110 true-positive cases, FSC testing yielded false-negative results. Blenden et al. [2] have previously reported that when mice were inoculated with street rabies virus, viral antigens were detected in muzzle skin before the development of clinical symptoms, then the antigen-positive rate increased with the progression of infection. In the present study, we could not clarify the reasons for the 3 false-negative results; however, it was concluded that the infection stage in individual dogs may have been a contributing factor. It is also possible that the number of FSCs used in the present study, 5, was not sufficient to yield 100% sensitivity, and that decomposition due to warm conditions in the field after death may have influenced the false-negative rate. Therefore, in order to establish the use of FSCs as a novel diagnostic method for canine rabies, further investigation is required.
Acknowledgments
The authors acknowledge the invaluable help of staff of the Pathology Department and Veterinary Research Department, Research Institute for Tropical Medicine (RITM), Department of Health, Filinvest Corporate City, Alabang, Muntinlupa City and the Regional Animal Disease Diagnostic Laboratory 3 (RADDL3), San Fernando City, Pampanga, Philippines for dog tissue collection and permission to use these samples for the current study. This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Kakenhi No. 26450410), the Grant for Scientific Research from the Kitasato University, Heiwa Nakajima Foundation, Japan Agency for Medical Research and Development (AMED) and AMED/JICA, SATREPS, Japan.
REFERENCES
- 1.Albas A., Ferrari C. I., da Silva L. H., Bernardi F., Ito F. H.1999. Influence of canine brain decomposition on laboratory diagnosis of rabies. Rev. Soc. Bras. Med. Trop. 32: 19–22. doi: 10.1590/S0037-86821999000100004 [DOI] [PubMed] [Google Scholar]
- 2.Blenden D. C., Bell J. F., Tsao A. T., Umoh J. U.1983. Immunofluorescent examination of the skin of rabies-infected animals as a means of early detection of rabies virus antigen. J. Clin. Microbiol. 18: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blenden D. C., Creech W., Torres-Anjel M. J.1986. Use of immunofluorescence examination to detect rabies virus antigen in the skin of humans with clinical encephalitis. J. Infect. Dis. 154: 698–701. doi: 10.1093/infdis/154.4.698 [DOI] [PubMed] [Google Scholar]
- 4.Boonsriroj H., Manalo D. L., Kimitsuki K., Shimatsu T., Shiwa N., Shinozaki H., Takahashi Y., Tanaka N., Inoue S., Park C. H.2016. A pathological study of the salivary glands of rabid dogs in the Philippines. J. Vet. Med. Sci. 78: 35–42. doi: 10.1292/jvms.15-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crepin P., Audry L., Rotivel Y., Gacoin A., Caroff C., Bourhy H.1998. Intravitam diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J. Clin. Microbiol. 36: 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimaano E. M., Scholand S. J., Alera M. T., Belandres D. B.2011. Clinical and epidemiological features of human rabies cases in the Philippines: a review from 1987 to 2006. Int. J. Infect. Dis. 15: e495–e499. doi: 10.1016/j.ijid.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 7.Fooks A. R., Banyard A. C., Horton D. L., Johnson N., McElhinney L. M., Jackson A. C.2014. Current status of rabies and prospects for elimination. Lancet 384: 1389–1399. doi: 10.1016/S0140-6736(13)62707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halata Z.1993. Sensory innervation of the hairy skin (light- and electronmicroscopic study. J. Invest. Dermatol. 101Suppl: 75S–81S. doi: 10.1016/0022-202X(93)90505-C [DOI] [PubMed] [Google Scholar]
- 9.Jackson A. C.2011. Update on rabies. Res. Rep. Trop. Med. 2: 31–43. doi: 10.2147/RRTM.S16013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OIE 2013. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th ed., pp. 1185–1191. World Organisation for Animal Health, Paris. [Google Scholar]
- 11.Sackett D. L.1992. The rational clinical examination. A primer on the precision and accuracy of the clinical examination. JAMA 267: 2638–2644. doi: 10.1001/jama.1992.03480190080037 [DOI] [PubMed] [Google Scholar]
- 12.Shimatsu T., Shinozaki H., Kimitsuki K., Shiwa N., Manalo D. L., Perez R. C., Dilig J. E., Yamada K., Boonsriroj H., Inoue S., Park C. H.2016. Localization of the rabies virus antigen in Merkel cells in the follicle-sinus complexes of muzzle skins of rabid dogs. J. Virol. Methods 237: 40–46. doi: 10.1016/j.jviromet.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 13.Shiwa N., Nakajima C., Kimitsuki K., Manalo D. L., Noguchi A., Inoue S., Park C. H.2018. Follicle sinus complexes (FSCs) in muzzle skin as postmortem diagnostic material of rabid dogs. J. Vet. Med. Sci. 80: 1818–1821. .doi: 10.1292/jvms.18-0519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji Y., Inoue S., Nakamichi K., Kurane I., Sakai T., Morimoto K.2004. Generation and characterization of P gene-deficient rabies virus. Virology 318: 295–305. doi: 10.1016/j.virol.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization 2013. WHO expert consultation on rabies. Second report. World Health Organ. Tech. Rep. Ser. 982: 1–139. [PubMed] [Google Scholar]


