Abstract
Background/purpose
Various chemical titanium (Ti) surface modifications have been reported for enhancing cellular activities that promote early osseointegration. The purpose of this study was to determine if sandblasted Ti coated with or without fibronectin (FN) or FN-derived peptides stimulated osteoblast-like cell adhesion, spreading, proliferation, and differentiation.
Materials and methods
Osteoblast-like cells (MC3T3-E1) were cultured on sandblasted Ti disks immobilized with FN or FN-derived peptides [GRGDSP (Gly-Arg-Gly-Asp-Ser), PHSRN (Pro-His-Ser-Arg-Asn), or GRGDSP/PHSRN]. Surface topography, cell morphology, cell adhesion, cell proliferation, analysis of osteogenesis-related genes and protein expression, alkaline phosphatase, and alizarin red staining of mineralization were evaluated.
Results
The sandblasted Ti coated with FN or FN-derived peptides enhanced cell adhesion and cell proliferation. However, the Ti coated with FN or FN-derived peptides groups were similar in cell spreading. Osteogenic differentiation was observed in the peptide-modified Ti surface groups, compared with that of the noncoated Ti group. FN and GRGDSP/PHSRN coating enhanced the gene and protein expression of Runx2, osteocalcin, and bone sialoprotein. Alkaline phosphatase activity and matrix mineralization were also markedly enhanced in the Ti coated groups.
Conclusion
The sandblasted Ti coated with FN or FN-derived peptides (GRGDSP/PHSRN) markedly enhance adhesion, proliferation, and differentiation of osteoblast-like cells compared with uncoated sandblasted Ti.
Keywords: fibronectin, GRGDSP, MC3T3-E1, PHSRN, titanium
Introduction
Various surface modifications of titanium (Ti) have been reported for enhancing osseointegration. The use of biomimetic molecules such as extracellular matrix (ECM) proteins, growth factors, or peptides immobilized on Ti surface promotes implant-host cell interactions.1 Fibronectin (FN), ECM-derived protein, and recombinant FN fragments coated on Ti improved biological performance of implants.2, 3 Nevertheless, these approaches might have limitations due to low stability of proteins, high cost of production, and immunogenicity.4 Therefore, modifying Ti surfaces with short synthetic peptides instead of using whole protein molecules may overcome the limitations as such. The RGD (Arg-Gly-Asp) peptide is the key attachment site for a variety of integrin receptors at the cell membrane, including α5β1 integrin. However, it has been proposed that, the PHSRN (Pro-His-Ser-Arg-Asn) is needed for a fully efficient cell attachment.5 Ti coated with the FN-derived peptide, GRGDSP (Gly-Arg-Gly-Asp-Ser-Pro), upregulated the mRNA levels of bone sialoprotein (BSP) and osteocalcin (OC) in osteoblast cells.6 A biomimetic peptide containing GRGDSP and PHSRN resulted in an increase in α5β1 adhesion when compared to pure GRGDSP.7 The colocalized RGD-PHSRN sequence improved osteoblast adhesion, spreading, and focal adhesion formation on polyethylene glycol hydrogel.8 Furthermore, the mixture of RGD-PHSRN promoted the attachment, spreading, and proliferation of osteogenic cells.9 Sandblasting-roughened Ti surfaces relatively enhanced early cell attachment compared to smooth surfaces in the presence of serum.10 The purpose of this study was to evaluate the effects of FN and FN-derived peptide immobilized on sandblasted Ti for osteogenic induction of osteoblast-like cells.
Materials and methods
Ti disks
Machined Grade 4 Ti (M-Ti) disks, 12 mm in diameter, were sandblasted with aluminum (Al2O3) particles 83 μm in size using a sandblasting machine (Pan Abrasives Ltd., Singapore, Singapore). After sandblasting, the samples (Ti) were cleaned by ultrasonication in acetone and then distilled water for 15 minutes each.
Surface analysis
The average surface roughness (Ra) was measured using a profilometer, Mitutoya SV-3000 (Mitutoya Co, Kanagawa, Japan). Disk topography and the residual aluminum were examined using a scanning electron microscope, Hitachi S-3400N (Hitachi Ltd., Tokyo, Japan) equipped with an energy dispersive X-ray spectrometer (n = 3, for each group).
Immobilization of FN and FN-derived peptides
The sandblasted Ti disks were immobilized with FN (FN-Ti), GRGDSP (GRGDSP-Ti), PHSRN (PHSRN-Ti), or GRGDSP/PHSRN (GRGDSP/PHSRN-Ti) using tresyl chloride (2,2,2-trifluoroethanesulfonyl chloride; Sigma-Aldrich, St. Louis, MO, USA).2, 6 The tresylated Ti disk surfaces were coated with 200 μL of human plasma FN (Harbor Bio-Products, Norwood, MA, USA), GRGDSP, PHSRN, or GRGDSP/PHSRN (50:50) solution at a concentration of 100 μg/mL each for 24 hours at 37°C. The coated disks were dried and stored in a desiccator. The noncoated sandblasted Ti disks served as the controls. The disks were sterilized using ethylene oxide (n = 3, for each group).
Confirmation of FN immobilization on the Ti disks
We determined the amount of FN immobilized on the sandblasted Ti disks using a modified enzyme-linked immunosorbent assay (ELISA). FN density was expressed as the amount of FN (ng)/area (cm2) on the Ti disks (n = 3, for each group).
Cell culture
The murine osteoblast cell line (MC3T3-E1) was cultured in a humidified atmosphere of 5% CO2 and maintained in α-MEM (Sigma Aldrich), supplemented with 10% heat-inactivated fetal bovine serum (Biowest, Kansas City, MO, USA), 100 μg/mL penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA), and 0.25 mg/mL amphotericin B (PAA Laboratories GmbH, Pasching, Austria). For the differentiation studies, osteoblast differentiation was induced with osteogenic medium (OM) containing 50 μg/mL ascorbic acid and 10mM β-glycerophosphate (Sigma Aldrich).
Cell adhesion and morphology assessment
To determine the optimal time to evaluate cell attachment, MC3T3-E1 cells were plated at a density of 105 cell/cm2 on 12 mm-sandblasted Ti disks coated with FN in 24-well tissue culture plates containing complete α-MEM medium. The number of unadherent cells was quantified after culturing for 10 minutes, 20 minutes, 30 minutes, or 60 minutes using a cell counter (Beckman Coulter, Fullerton, CA, USA). The number of attached cells was then calculated. The morphological characteristics of the attached cells were determined using a scanning electron microscope (n = 3, for each group). We observed the sequential changes in appearances of attached cells from rounding cells with few cytoplasmic extensions to cells with focal cytoplasmic extension, circumferential spreading, and full spreading and flattening into polygonal shapes.11
Cell proliferation assessment
Cell proliferation was analyzed using the Alamar Blue cell viability assay kit (AbD Serotec, Oxford, UK).12 Cell proliferation was measured at 3 days, 5 days, 7 days, 10 days, and 14 days after initially seeding 1 × 105 MC3T3-E1 cells on the surfaces of the treated and untreated Ti disks. The samples were washed with phosphate-buffered saline (PBS) and incubated with the Alamar blue solution for another 4 hours. The reaction medium was then spectrophotometrically measured at 570 nm and 600 nm using a microplate reader (Tecan Group Ltd., Mannerdorf, Switzerland). The results were expressed as a percentage of Alamar Blue reduction per the manufacturer's instructions (n = 3, for each group).
Real-time polymerase chain reaction (PCR) analysis
The expression of osteogenesis-related genes in the MC3T3-E1 cells was determined after culturing on experimental disks for 14 days. Total RNA of adherent cells was extracted using TRIzol reagent (Thermo Fisher Scientific). The RNA samples were reversed-transcribed into cDNA using the Primescript RT reagent kit (Takara Bio Inc., Shiga, Japan). The expression of runt-related transcription factor 2 (Runx2), OC, and BSP was determined by a real time PCR machine (iQ 5, Bio-Rad, Hercules, CA, USA) with the QuantiTest SYBR Green kit (Qiagen, Hilden, Germany). The primer sequences are listed in Table 1. A negative control without a cDNA template was run in each assay. The data were analyzed using relative expression analysis (2−ΔΔCt).13 Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control (n = 3 for each group).
Table 1.
Forward (F) and reverse (R) sequence of primers used in the real-time polymerase chain reaction (PCR) of targets and housekeeping genes.
| Gene | Primer sequence (5′→3′) | Amplicon size (base pairs) |
|---|---|---|
| Runx2 | (F) CTCAGTGATTTAGGGCGCATT (R) AGGGGTAAGACTGGTCATAGG |
178 |
| OC | (F) AGCTATCAGACCAGTATGGCT (R) TTTTGGAGCTGCTGTGACATC |
180 |
| BSP | (F) TACCGAGCTTATGAGGACGAA (S) GCATTTGCGGAAATCACTCTG |
246 |
| GAPDH | (F) ATCACCATCTTCCAGGAG (S) ATCGACTGTGGTCATGAG |
318 |
BSP = bone sialoprotein; GADPH = glyceraldehyde-3-phosphate dehygrogenase; OC = osteocalcin.
Western blotting analysis
Protein expression of the target genes was examined by Western blot. Total protein in the cell lysates was determined using the BCA Protein Assay Kit (Thermo Fisher Scientific) as per the manufacturer's instructions. Equal amounts of protein were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to nitrocellulose membranes and then incubated with anti-Runx2 (M-70, Santa Cruz Biotechnology Inc., Dallas, TX, USA; 1:500 dilution), anti-OC (FL-95, Santa Cruz; 1:500 dilution), anti-BSP (H-157, Santa Cruz; 1:500 dilution), or anti-β-actin (N-21, Santa Cruz; 1:500 dilution) antibody. The membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibody (Sc-2004, Santa Cruz; 1:40,000 dilution). Immunoreactive proteins were detected using a SuperSignal West Pico Chemiluminescent Substrate blotting kit (Thermo Fisher Scientific). Immunoreactive bands were visualized using chemiluminescence (Syngene GeneGnome, Synoptics Ltd., Cambridge, UK). The relative values of the protein bands were normalized to the internal control β-actin bands (n = 3, for each group).
Assessment of cell differentiation
MC3T3-E1 cells were seeded onto treated and nontreated Ti disk samples and incubated in OM. Cells were harvested at Day 3, Day7, and Day14. Briefly, after lysing the cells with radioimmunoprecipitation assay (RIPA) buffer, alkaline phosphatase (ALP) activity was determined at the indicated time points using the ALP Kit (Teco Diagnostic, Anaheim, CA, USA) as per the manufacturer's protocol. The protein concentration of lysates was determined using the BCA Protein Assay Kit (Thermo Fisher Scientific) The specific activity of ALP was calculated in reference to the protein concentration of lysates and expressed as units/mg protein.
Mineralization was evaluated by Alizarin red staining after culturing MC3T-E1 cells on plastic and differently treated Ti surfaces for 7 days and 14 days in OM.14 The cells were stained with 2% Alizarin Res S (Sigma-Aldrich) for 10 minutes at room temperature. The mineralized nodules were observed under a stereo microscope (Zeiss Stemi 2000 C, Carl Zeiss, Oberkochen, Germany).
Statistical analysis
The data were expressed as mean ± standard error of the mean (SEM). The analysis of variance (ANOVA) test with Bonferroni's posttest was performed using Prism GraphPad 6 software (Graphpad Software Inc., La Jolla, CA, USA) to assess statistically significant differences (P ≤ 0.05) between the groups.
Results
Surface topography and roughness
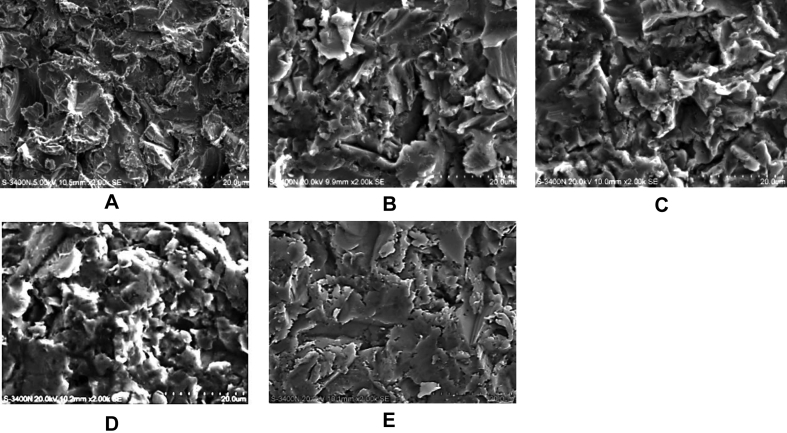
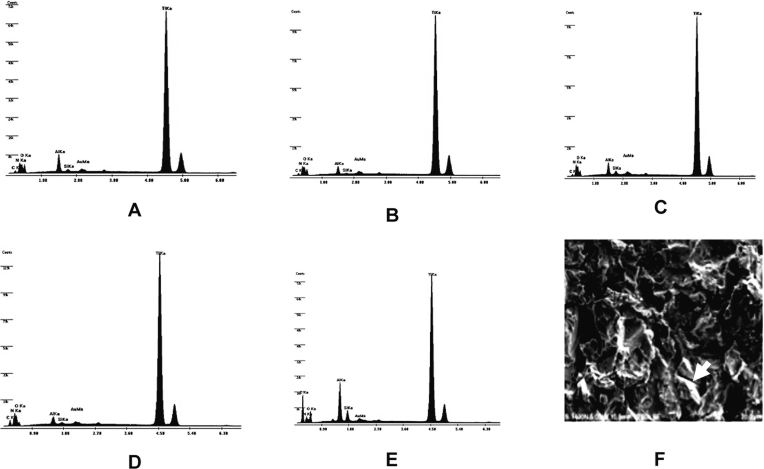
The sandblasted Ti surfaces demonstrated a rough and irregular pattern (Figure 1A). A reticulated appearance with undermining deformation was observed on the surface. No difference in surface morphology was observed on the differently treated Ti disks (Figures 1B–1E). Energy dispersive X-ray (EDX)-surface analysis (Figures 2A–2E) revealed the presence of essentially Ti surfaces for all groups. However, additional elements such as Al, C, O, Si, N, and Au were found in the surfaces. Scanning electron micrographs (SEM; Figure 2F) also showed that residual Al2O3 particles were present on the Ti surface. Surface analysis by a profilometer revealed a significant increase in Ra for sandblasted Ti (2.24 ± 0.22 μm) compared with M-Ti (0.6 ± 0.03 μm; P < 0.05).
Figure 1.
Topographic analyses of differently treated Ti surfaces. Scanning electron microscopy (SEM) of (A) Ti, (B) FN-Ti, (C) GRGDSP-Ti, (D) PHSRN-Ti, and (E) GRGDSP/PHSRN-Ti.
Figure 2.
Energy dispersive X-ray (EDX) of experimental Ti disks. Typical EDX spectrum observed on (A) Ti, (B) FN-Ti, (C) GRGDSP-Ti, (D) PHSRN-Ti, and (E) GRGDSP/PHSRN-Ti. Profiles of EDX show the characteristic peaks of Ti and Al. (F) A representative scanning electron micrograph (SEM) demonstrates residual alumina particles (arrow) on the experimental Ti disk.
FN immobilization and morphology of adherent cells
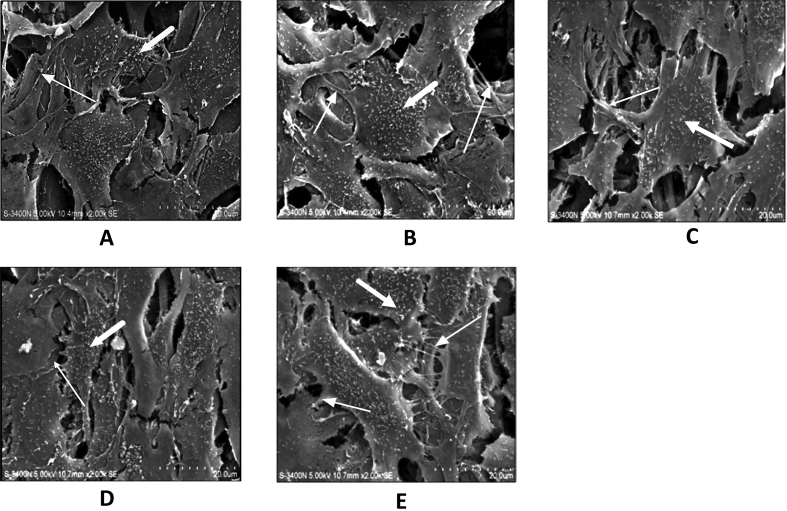
We observed that FN-coated Ti contained FN at 776.02 ng/cm2. This density represents ∼5.14% of the total FN amount incubated with the Ti disks. Cells attached on differently treated Ti surfaces (Figure 3) showed irregular flat-shaped cells with cytoplasmic extensions and short-fiber-like structure, which is presumed to be filopodia and microvilli-like structures, respectively.15 Immobilized FN (Figure 3B) or FN-derived peptides (Figures 3C–E) did not have any influence on the morphology of adherent cells as compared to that of uncoated Ti surfaces (Figure 3A).
Figure 3.
Morphology of adherent osteoblasts on differently treated Ti surfaces. Scanning electron microscopy (SEM) of MC3T3-E1 cells cultured on (A) Ti, (B) FN-Ti, (C) GRGDSP-Ti, (D) PHSRN-Ti, and (E) GRGDSP/PHSRN-Ti for 2 days. Attached cells show cytoplasmic extensions, presumed to be microvilli (large arrows) and filopodia-like structures (thin arrows).
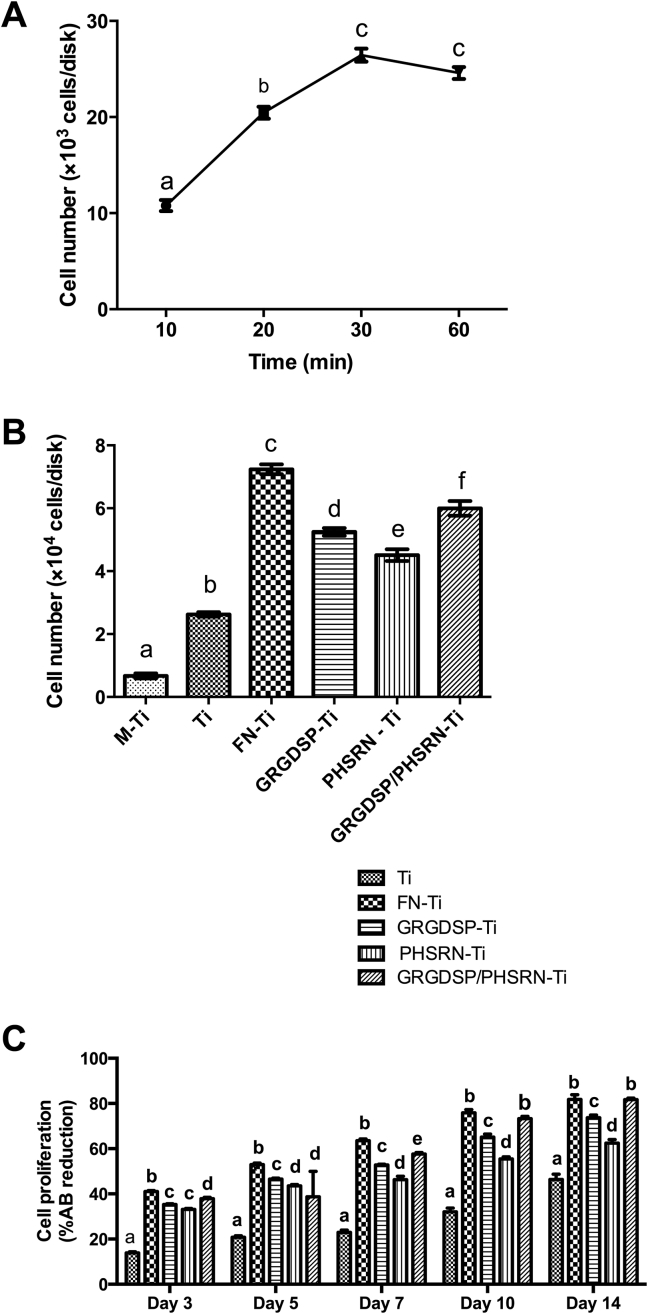
Cell adhesion and proliferation
Our preliminary studies revealed that the number of adherent cells on sandblasted Ti coated with FN gradually increased after 30 minutes (Figure 4A). Therefore, incubation for 30 minutes was used to study cell adhesion in further experiments. Cell adhesion was significantly enhanced in the FN-Ti group, followed by the GRGDSP/PHSRN-Ti, GRGDSP-Ti, PHSRN-Ti, and Ti groups, compared with the M-Ti group (P < 0.05; Figure 4B). A significant increase in cell proliferation was found when Ti was coated with FN or FN derivative peptides, compared with uncoated Ti (P < 0.05; Figure 4C). The results were comparable for Day 3, Day 5, Day 7, Day 10, and Day 14.
Figure 4.
Cell adhesion and cell proliferation of adherent osteoblasts on differently treated Ti surfaces. (A) Adhesion of MC3T3-E1 cells on FN-Ti after 10 minutes, 20 minutes, 30 minutes, and 60 minutes of culture. (B) Cell adhesion of MC3T3-E1 cells on differently treated Ti surfaces for 30 minutes. (C) Cell proliferation of MC3T3-E1 cells determined by an Alamar Blue assay at Day 3, Day 5, Day 7, Day 10, and Day 14. Values represent the mean ± SEM from three separate experiments. The data were analyzed by analysis of variance. Different superscript letters in A and B denote a significant difference based on Bonferroni's posttest (P < 0.05). Bars with different superscript letters in C indicate a significant difference (Bonferroni's posttest, P < 0.05) when compared in the same treatment group.
Cell differentiation markers
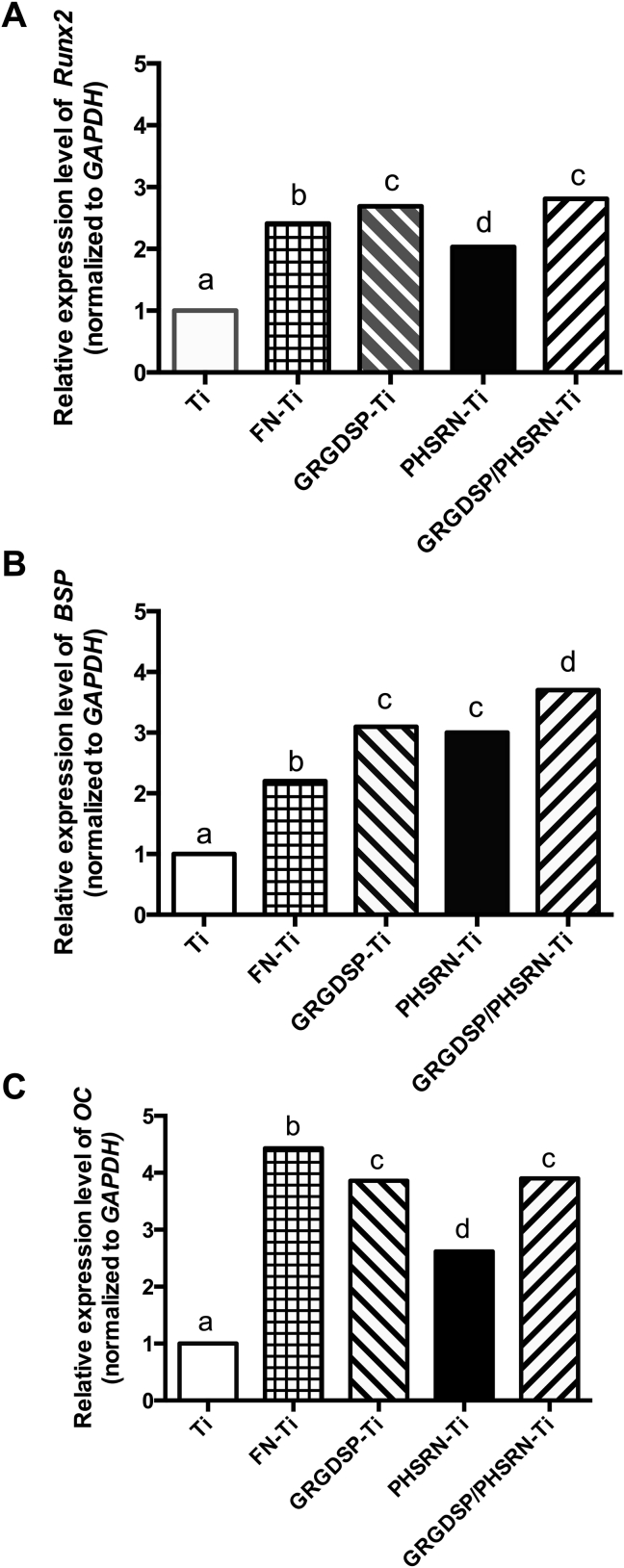
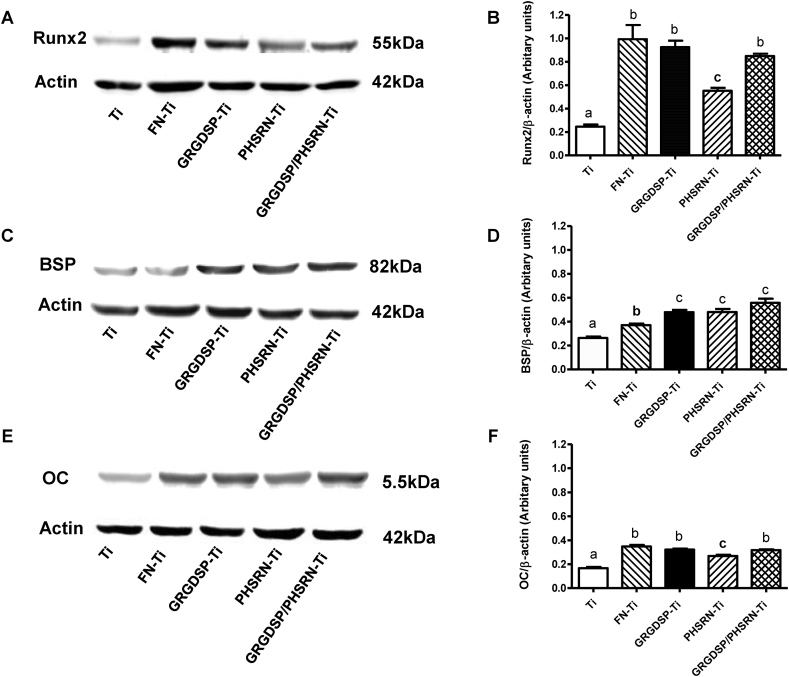
FN and FN-derived peptides significantly upregulated Runx2, BSP, and OC after culturing for 14 days (P < 0.05). Marked upregulation was observed in the FN and GRGDSP/PHSRN coated groups (Figures 5A–5C). The protein expression profile was consistent with the osteogenic gene upregulation (Figures 6A–C).
Figure 5.
Gene expression of (A) Runx2, (B) bone sialoprotein (BSP), and (C) osteocalcin (OC) in MC3T3-E1 cells at day 14 on differently treated Ti surfaces. Gene expression was determined by real-time polymerase chain reaction (PCR). Ratios of target genes were normalized to the housekeeping gene (GAPDH). Relative expression levels represent fold changes of gene expression in MC3T3-E1 cells on coated Ti surfaces compared to that on uncoated Ti surfaces. Values represent the mean ± SEM from three separate experiments. The data were analyzed by analysis of variance. Bars with different superscript letters denote a significant difference based on Bonferroni's post-test (P < 0.05).
Figure 6.
The expression of bone-specific proteins of MC3T3-E1 cells at Day 14 on differently treated Ti surfaces. The Runx2 (A), bone sialoprotein (BSP) (C), and osteocalcin (OC) (E) protein levels were detected using Western blotting, with β-actin as the loading control (Left). Right shows graphic representations of densitometric quantitation of the Western blotting signals of Runx2 (B), BSP (D), and OC (F). The intensity of each protein band was normalized to the β-actin band. Values represent the mean ± SEM from three separate experiments. The data were analyzed by analysis of variance. Bars with different superscript letter denote a significant difference based on Bonferroni's post-test (P < 0.05).
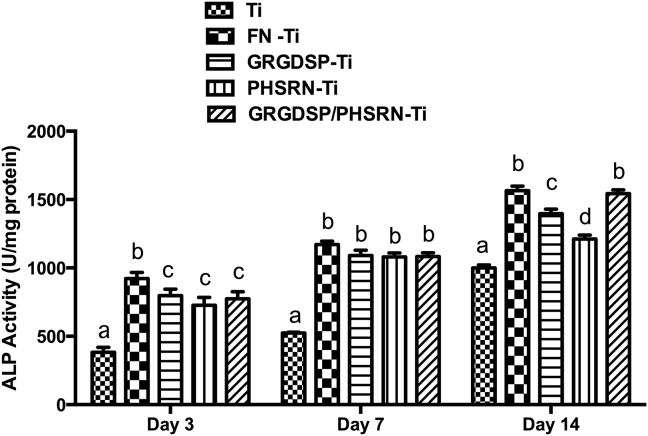
ALP activity levels were significantly increased in cells on Ti coated with FN or its derivative peptides at Day 3, Day 7, and Day 14, compared to that of uncoated Ti (P < 0.05) (Figure 7). The FN and GRGDSP/PHSRN groups exhibited the highest ALP activities at Day 14. However, there was no significant difference in ALP activities between the two groups (P > 0.05).
Figure 7.
Alkaline phosphatase (ALP) activities of MC3T3-E1 grown on differently treated Ti surfaces. MC3T3-E1 cells were cultured on differently treated Ti disks for 3 days, 7 days, or 14 days. ALP activity was determined in cell lysates at indicated time points. Values represent the mean ± SEM from three separate experiments. Data were analyzed by analysis of variance. Bars with different superscript letters denote a significant difference based on Bonferroni's post-test (P < 0.05) when compared in the same treatment group.
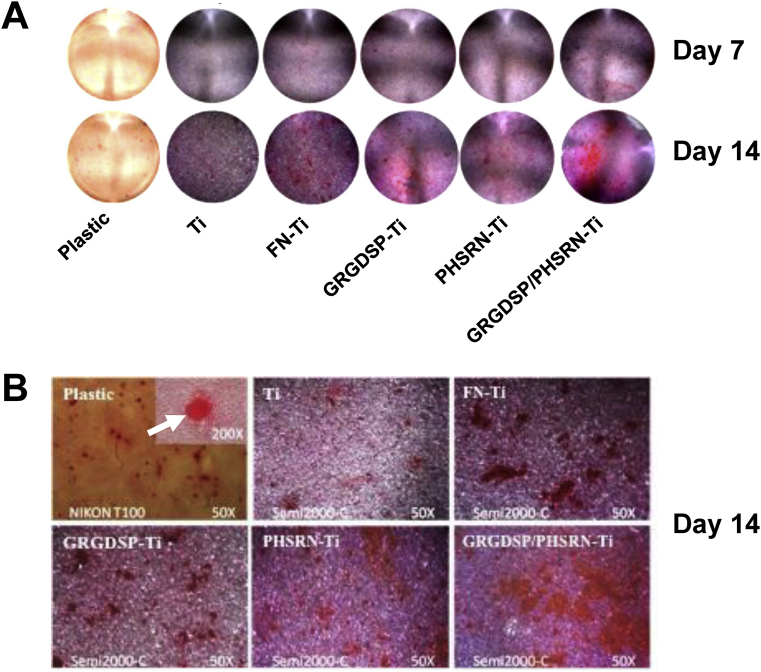
Alizarin red staining shows mineralization of ECMs after culturing MC3T3-E cells on plastic and differently treated Ti surfaces for 7 days and 14 days in osteogenic media. All of the surfaces showed increased signs of mineral deposits over time (Figure 8A). Generally, an increase in matrix mineralization was obviously observed in the coated Ti groups, compared to uncoated Ti groups (Figures 8A and 8B). Coating with FN and GRGDSP/PHSRN exhibited the great degrees of stains. Plastic surfaces showed the least amount of mineral deposits among all of the surfaces.
Figure 8.
Biomineralization assay using Alizarin red staining after 7 days and 14 days of cell proliferation and differentiation. (A) Alizarin red staining shows mineralization in extracellular matrix of MC3T3-E1 cells grown on plastic and differently treated Ti surfaces for 7 days and 14 days. (B) High magnifications of Alizarin red staining of MC3T-E1 cells, which were cultured on the differently treated surfaces for 14 days. The inset shows a representative mineralized nodule (arrow).
Discussion
In the present study, SEM analysis revealed rough surfaces on sandblasted Ti disks. Our micrographic images are similar to those of previous reports.16, 17 Based on the energy dispersive X-ray spectrometry analysis, Al and O were detected on the sandblasted Ti surfaces, demonstrating the presence of remaining Al2O3 particles, as also observed by Almilhatti et al.18 Other contaminants including C, N, Si, and Au were also found in commercial Ti grade 4.19, 20
The surface roughness (Ra) analysis performed in our study corresponded with that of a previous report.21 The range of surface roughness in our samples is commonly found in oral implants22 and it was appropriate for in vitro biologic response.21 A roughened surface is essential in cell adhesion for cell anchorage, and roughness enhances cell mobility and podia formation.23
Although the amount of immobilized GRGDSP and/or PHSRN peptides was not directly determined in the present study, we used a relatively high concentration of the peptides (100 μg/mL) compared to that of the study of Yamamichi et al.6 The authors demonstrated that GRGDSP was bound to Ti surfaces after coating with GRGDSP at 100μM (58.75 μg/mL) using tresyl chloride activation. Therefore, we assumed that GRGDSP and GRGDSP and/or PHSRN were bound to the Ti surfaces via tresyl chloride in our model.
The binding of cells to the ECM generates intracellular signaling that affects the cytoskeleton, initiates actin microfilament and focal adhesion assembly, and influences cell spreading.24, 25, 26, 27 In our study, FN and FN-derived peptides remarkably enhanced osteoblast-like cell attachment, proliferation, and differentiation, which is consistent with previous reports.2, 6, 15, 28 Integrins might play an important role in FN binding through the RDG sequences in FN.29
We demonstrated that the adhesion of MC3T3-E1 cells was markedly enhanced in the FN-Ti group, followed by the GRGDSP/PHSRN-Ti, GRGDSP-Ti, PHSRN-Ti, Ti, and M-Ti groups. The GRDGSP group might have the highest binding activity to MC3T3-E1cells compared to that of RGD-motif peptides derived from other ECM proteins.30 Greater cell polarization or elongation was found when bone marrow mesenchymal cells attached to silanized Ti coated with GRGDSP compared to uncoated Ti.23 In addition, the colocalized RGD-PHSRN sequences improved osteoblast adhesion, spreading, and focal adhesion formation when compared to RGD alone.8 Hydrophilic substrates coated with PHSRN enhanced human fibroblast spreading.31 Furthermore, GRGDSP and PHSRN also supported integrin-mediated cell adhesion.7, 32, 33 It is suggested that GRGDSP is the primary recognition sequence for α5β1, whereas PHSRN is the synergy-binding site for this integrin.34 Therefore, it is possible that in our study GRGDSP/PHSRN coating enhances α5β1 integrin binding sites.3
Hilbig et al35 demonstrated upregulation of OC expression in bone cells that were cultured on FN coated Ti. As expected, we found that MC3T3-E1 cells enhanced OC expression on coated Ti, compared with noncoated Ti. In our study, BSP expression was notably increased in the FN-Ti group, which is consistent with previous studies.6 Runx2 has been shown to regulate OC.36 We demonstrated that both the mRNA and protein levels of Runx2 were enhanced in the coated Ti groups. This suggests that FN and FN-derived peptides promote osteogenic differentiation by upregulating Runx2. FN coated Ti induced ALP activity in osteoblast cells.29, 37 However, Yoshida et al38 reported that FN immobilization did not remarkably enhance cell proliferation, ALP activity, OC production or matrix mineralization in MC3T3-E1cells. By contrast, other studies have shown that osteoblast cells grown on Ti coated with GRGDSP or PHSRN expressed significantly higher levels of OC, BSP, ALP, and matrix mineralization.3, 8, 39 The relationship between ALP and OC was previously reported in a study of differentiation associated genes (ALP and OC) based on the mRNA levels expressed by calvarial-derived osteoblasts.40 Taken together, our findings indicate that modified sandblasted Ti surfaces with FN or FN-derived peptides enhance osteogenic induction. Coating sandblasted Ti with FN or GRGDSP/PHSRN exerts a greater effect on the stimulation of osteogenic differentiation in MC3T3-E1cells compared to sandblasted Ti modified with individual peptide.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by Thammasat University, Klongluang, Prathumtani, Thailand (Grant No. TU 2556). The authors acknowledge Dr. Yasuko Shibata and Dr. Yoshimitsu Abiko for providing the MC3T3-E1 cell line and Dr. Lertrit Sarinnaphakorn for technical help in Alamar Blue assays.
References
- 1.Avila G., Misch K., Galindo-Moreno P., Wang H.L. Implant surface treatment using biomimetic agents. Implant Dent. 2009;18:17–26. doi: 10.1097/ID.0b013e318192cb7d. [DOI] [PubMed] [Google Scholar]
- 2.Pugdee K., Shibata Y., Yamamichi N. Gene expression of MC3T3-E1 cells on fibronectin-immobilized titanium using tresyl chloride activation technique. Dent Mater J. 2007;26:647–655. doi: 10.4012/dmj.26.647. [DOI] [PubMed] [Google Scholar]
- 3.Petrie T.A., Raynor J.E., Reyes C.D., Burns K.L., Collard D.M., Garcia A.J. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials. 2008;29:2849–2857. doi: 10.1016/j.biomaterials.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier J.H., Segura T. Evolving the use of peptides as components of biomaterials. Biomaterials. 2011;32:4198–4204. doi: 10.1016/j.biomaterials.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson S., Svineng G., Wennerberg K., Armulik A., Lohikangas L. Fibronectin-integrin interactions. Front Biosci. 1997;2:d126–d146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 6.Yamamichi N., Pugdee K., Chang W.J. Gene expression monitoring in osteoblasts on titanium coated with fibronectin-derived peptide. Dent Mater J. 2008;27:744–750. doi: 10.4012/dmj.27.744. [DOI] [PubMed] [Google Scholar]
- 7.Mardilovich A., Kokkoli E. Biomimetic peptide-amphiphiles for functional biomaterials: the role of GRGDSP and PHSRN. Biomacromolecules. 2004;5:950–957. doi: 10.1021/bm0344351. [DOI] [PubMed] [Google Scholar]
- 8.Benoit D.S., Anseth K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209–5220. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 9.Mas-Moruno C., Fraioli R., Albericio F., Manero J.M., Gil F.J. Novel peptide-based platform for the dual presentation of biologically active peptide motifs on biomaterials. ACS Appl Mater Interfaces. 2014;6:6525–6536. doi: 10.1021/am5001213. [DOI] [PubMed] [Google Scholar]
- 10.Nishimoto S.K., Nishimoto M., Park S.W. The effect of titanium surface roughening on protein absorption, cell attachment, and cell spreading. Int J Oral Maxillofac Implants. 2008;23:675–680. [PubMed] [Google Scholar]
- 11.Lumbikanonda N., Sammons R. Bone cell attachment to dental implants of different surface characteristics. Int J Oral Maxillofac Implants. 2001;16:627–636. [PubMed] [Google Scholar]
- 12.Yu X., Walsh J., Wei M. Covalent immobilization of collagen on titanium through polydopamine coating to improve cellular performances of MC3T3-E1 cells. RSC Adv. 2013;4:7185–7192. doi: 10.1039/C3RA44137G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finke B., Luethen F., Schroeder K. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials. 2007;28:4521–4534. doi: 10.1016/j.biomaterials.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Jeong Y., Yang W., Ko H., Kim M. The effects of bone morphogenetic protein-2 and enamel matrix derivative on the bioactivity of mineral trioxide aggregate in MC3T3-E1cells. Restor Dent Endod. 2014;39:187–194. doi: 10.5395/rde.2014.39.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa T., Yoshida E., Yoshimura Y., Uo M., Yoshinari M. MC3T3-E1 Cells on titanium surfaces with nanometer smoothness and fibronectin immobilization. Int J Biomater. 2012;2012 doi: 10.1155/2012/743465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degasne I., Basle M.F., Demais V. Effects of roughness, fibronectin and vitronectin on attachment, spreading, and proliferation of human osteoblast-like cells (Saos-2) on titanium surfaces. Calcif Tissue Int. 1999;64:499–507. doi: 10.1007/s002239900640. [DOI] [PubMed] [Google Scholar]
- 17.Keller J.C., Schneider G.B., Stanford C.M., Kellogg B. Effects of implant microtopography on osteoblast cell attachment. Implant Dent. 2003;12:175–181. doi: 10.1097/01.id.0000058309.77613.87. [DOI] [PubMed] [Google Scholar]
- 18.Almilhatti H.J., Neppelenbroek K.H., Vergani C.E., Machado A.L., Pavarina A.C., Giampaolo E.T. Adhesive bonding of resin composite to various titanium surfaces using different metal conditioners and a surface modification system. J Appl Oral Sci. 2013;21:590–596. doi: 10.1590/1679-775720130255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castilho G.A.A., Martins M.D., Macedo W.A.A. Surface characterization of titanium based dental implants. Braz J Phys. 2006;36:1004–1008. [Google Scholar]
- 20.Jorge J.R., Barao V.A., Delben J.A., Faverani L.P., Queiroz T.P., Assuncao W.G. Titanium in dentistry: historical development, state of the art and future perspectives. J Indian Prosthodont Soc. 2013;13:71–77. doi: 10.1007/s13191-012-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M.J., Kim C.W., Lim Y.J., Heo S.J. Microrough titanium surface affects biologic response in MG63 osteoblast-like cells. J Biomed Mater Res A. 2006;79:1023–1032. doi: 10.1002/jbm.a.31040. [DOI] [PubMed] [Google Scholar]
- 22.Albrektsson T., Wennerberg A. Oral implant surfaces: Part 2–review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004;17:544–564. [PubMed] [Google Scholar]
- 23.Chen W.C., Ko C.L. Roughened titanium surfaces with silane and further RGD peptide modification in vitro. Mater Sci Eng C Mater Biol Appl. 2013;33:2713–2722. doi: 10.1016/j.msec.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 24.Pankov R., Yamada K.M. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 25.Larsen M., Artym V.V., Green J.A., Yamada K.M. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Meredith J.E., Jr., Winitz S., Lewis J.M. The regulation of growth and intracellular signaling by integrins. Endocr Rev. 1996;17:207–220. doi: 10.1210/edrv-17-3-207. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K.M., Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- 28.Petrie T.A., Reyes C.D., Burns K.L., Garcia A.J. Simple application of fibronectin-mimetic coating enhances osseointegration of titanium implants. J Cell Mol Med. 2009;13:2602–2612. doi: 10.1111/j.1582-4934.2008.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pegueroles M., Aguirre A., Engel E. Effect of blasting treatment and Fn coating on MG63 adhesion and differentiation on titanium: a gene expression study using real-time RT-PCR. J Mater Sci Mater Med. 2011;22:617–627. doi: 10.1007/s10856-011-4229-3. [DOI] [PubMed] [Google Scholar]
- 30.Abiko Y., Pugdee K., Hirai T., Chu C., Ando T., Shibata Y. Attachment activity of RGD-motif peptides to osteoblast-lie cell line MC3T3-E1. Int J Oral Med Sci. 2007;6:97–99. [Google Scholar]
- 31.Satriano C., Messina G.M., Marino C. Surface immobilization of fibronectin-derived PHSRN peptide on functionalized polymer films–effects on fibroblast spreading. J Colloid Interface Sci. 2010;341:232–239. doi: 10.1016/j.jcis.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 32.Ochsenhirt S.E., Kokkoli E., McCarthy J.B., Tirrell M. Effect of RGD secondary structure and the synergy site PHSRN on cell adhesion, spreading and specific integrin engagement. Biomaterials. 2006;27:3863–3874. doi: 10.1016/j.biomaterials.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y., Mrksich M. The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry. 2004;43:15811–15821. doi: 10.1021/bi049174+. [DOI] [PubMed] [Google Scholar]
- 35.Hilbig H., Kirsten M., Rupietta R. Implant surface coatings with bone sialoprotein, collagen, and fibronectin and their effects on cells derived from human maxillar bone. Eur J Med Res. 2007;12:6–12. [PubMed] [Google Scholar]
- 36.Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 37.Ren X., Wu Y., Cheng Y., Ma H., Wei S. Fibronectin and bone morphogenetic protein-2-decorated poly(OEGMA-r-HEMA) brushes promote osseointegration of titanium surfaces. Langmuir. 2011;27:12069–12073. doi: 10.1021/la202438u. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida E., Yoshimura Y., Uo M., Yoshinari M., Hayakawa T. Influence of nanometer smoothness and fibronectin immobilization of titanium surface on MC3T3-E1 cell behavior. J Biomed Mater Res A. 2012;100:1556–1564. doi: 10.1002/jbm.a.34084. [DOI] [PubMed] [Google Scholar]
- 39.Viereck V., Siggelkow H., Tauber S., Raddatz D., Schutze N., Hufner M. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem. 2002;86:348–356. doi: 10.1002/jcb.10220. [DOI] [PubMed] [Google Scholar]
- 40.Owen T.A., Aronow M., Shalhoub V. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]