CD19 is widely used as a marker of B-cell lineage because it is expressed as early as the pre-B stage and repressed only during terminal differentiation into plasma cells.1 This transmembrane glycoprotein is an essential co-receptor of the B-cell receptor (BCR),2 although it can also be activated in a BCR-independent manner.3 The deleterious consequences of CD19 inactivation on B-cell development, described both in murine models and in human diseases,4,5 are explained by the potential of CD19 to activate pro-survival pathways transduced by phosphatidylinositol-3-kinase (PI3K) or kinases from the Src family. Accordingly, the expression of CD19 is highly conserved in B-cell neoplasms, except in those derived from terminally differentiated cells such as plasmablastic lymphoma and multiple myeloma.6 Of note, CD19 has become a therapeutic target for bispecific antibodies (blinatumomab) or chimeric antigen receptor (CAR) T cells in B-cell malignancies.7
We report the case of a patient diagnosed with diffuse large B-cell lymphoma (DLBCL) that did not express CD19 as assessed by flow cytometry and describe the molecular mechanisms that explain this exceptional observation.
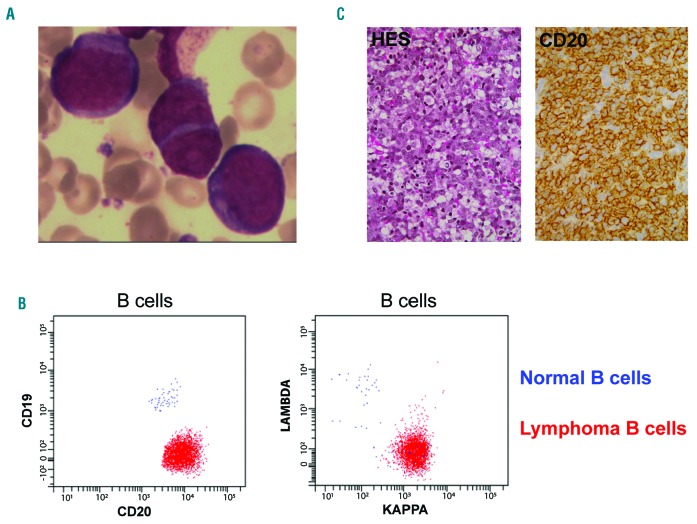
A 76-year-old woman was hospitalized for asthenia, persistent fever, and night sweats. Physical examination was normal except for massive splenomegaly (craniocaudal length of 20 cm on CT-scan). Analysis of blood and bone marrow aspiration smears detected around 20% atypical lymphoid cells with uncondensed chromatin, visible nucleoli in some cells, and moderately basophilic cytoplasm (Figure 1A). On immunophenotyping these cells strongly expressed CD45 together with B-cell differentiation markers (CD20, CD79b, and CD22) and showed a monotypic kappa light chain expression. However, they were negative for the CD19 antigen, whereas the residual normal B-cells were CD19 positive, as expected (Figure 1B). The lymphoma cells expressed MUM1, but no marker of plasma cell differentiation (CD38, CD138), and they did not express the ALK protein. A bone marrow biopsy confirmed the diagnosis of diffuse large B-cell lymphoma (Figure 1C) of the activated-B-cell subtype as assessed by reverse transcription multiplex ligation-dependent probe amplification.8 Additional cytogenetic analysis by fluorescent in situ hybridization on paraffin-embedded sections showed an additional 3q27 locus (BCL6) and did not identify rearrangements of cMYC, BCL2 or CCND1 loci. According to the international prognostic index, this patient was at clinical stage 5 (elevated LDH (840 IU, normal range (240-480), ECOG status = 3, age above 60, extra-nodal involvement (bone marrow), stage IV). HIV serology was negative. She was treated with 8 R-CHOP associated with intrathecal methotrexate prophylaxis. PET scan showed complete metabolic remission after 4 cycles and this finding was confirmed at the end of the treatment (6-month follow up).
Figure 1.
Diagnosis of a CD19-negative DLBCL. (A) Lymphoma cells in bone marrow smears (MGG staining, ×500 magnification). (B) Flow cytometry analysis of the lymphoma cells (red dots) and residual normal B-cells (blue dots) in the blood, after staining of CD19 and CD20 (left) and kappa and lambda light chains (right). (C) Bone marrow biopsy showing infiltration by the DLBCL (left panel: hematoxylin eosin saffron staining, ×20 magnification, right panel, CD20 staining, ×40 magnification).
In order to understand the unexpected negative results for CD19 expression, lymphoma cells were purified by CD20 cell sorting (RoboSepTM, StemCell Technologies, Vancouver, Canada). Comparative genomic hybridization with single nucleotide polymorphism array (SurePrint G3 Cancer CGH+SNP Microarray Kit, 4x180K, Agilent, Santa Clara, CA, USA) found an uniparental disomy involving the short arm of chromosome 16, which encompasses the locus coding for CD19 (16p11.2; Figure 2A). Sanger sequencing of the CD19 gene performed on germline DNA (CD20-negative fraction) did not find any abnormalities, whereas a homozygous splice mutation (c.560-1G>C, NM_001178098.1) was detected in the lymphoma cells (Figure 2B). In order to evaluate the transcriptional consequences of this mutation in the acceptor site of intron 3 (between exons 3 and 4), we extracted RNA from the FFPE bone marrow biopsy after DNAse digestion (Maxwell 16 Lev RNA FFPE kit (Promega)). We performed reverse transcription followed by polymerase chain reaction with a forward primer on exon 3 (GACAGCCTGAACCAGAGCCT) and reverse primer on exon 4 (CTAGGCTCAGCAATGACTTAG). PCR conditions were: initial denaturation (94°C, 2 min), 35 cycles of denaturation (94°C, 30 sec), hybridization (60°C, 30 sec) and elongation (72°C, 30 sec), and a final step of 2 min at 72°C. Patient’s amplicon size was larger than that found from a control sample (Figure 2C), and we confirmed by Sanger sequencing that this was due to the retention of intron 3. Importantly, genomic DNA contamination was ruled out because this band was not observed when the PCR was done on mRNA, performing the reverse transcription step without reverse transcriptase. As a consequence, a premature stop codon occurs 23 amino-acids later, which explains the absence of CD19 expression in this case of DLBCL (Figure 2D). It should be noted that we only assessed CD19 expression at the membrane surface, thus we cannot rule out intracellular expression of a truncated CD19 protein which could activate signaling pathways.
Figure 2.
Molecular mechanisms of CD19 inactivation. (A) Single-nucleotide polymorphism analysis showing a uniparental disomy of the short arm of chromosome 16. (B) Sanger sequencing of the CD19 gene showing no abnormality in the germline material (left) and a homozygous mutation of the splice acceptor site of intron 3 (c.560-1G>C, NM_001178098.1) in the DLBCL cells (right). (C) Polymerase chain reaction analysis using primers on exons 3 and 4 of CD19, showing the same amplicon size in genomic DNA and in cDNA from the DLBCL diagnosis sample because of retention of intron 3. Note that no amplification occurs when using a non-reverse-transcribed RNA matrix, thus ruling out genomic DNA contamination. The cDNA from a control patient (CTR) with CD19-positive DLBCL shows a smaller amplicon because the intron 3 was spliced. A schematic representation of the experiment is provided (right). (D) Schematic overview of the molecular mechanisms explaining this CD19-negative DLBCL. Note that we did not rule out intracellular expression of CD19 (right panel, bottom).
As CD19 transduces pro-survival signals, the observation of a DLBCL displaying loss of CD19 expression at the time of diagnosis is unexpected. CD19 negativity is usual in plasmablastic lymphomas that are derived from B-cells committed to plasmacytic differentiation. However, the whole phenotypic profile of these lymphomas reflects this stage of differentiation, with expression of plasma cell markers (such as CD138 or CD38) and loss of expression of all B-cell markers such as CD20 and CD196. The patient described here did not express any plasma cell markers, and did not repress the other B-cell markers such as CD20 or CD79b, thus ruling out a diagnosis of plasmablastic lymphoma. Loss of B-cell markers has also been described in ALK-positive DLBCL, but the case described here was negative for ALK protein. Regarding DLBCL, some cases have been described as CD19 negative using immunohistochemistry,9–11 but this was not confirmed by flow cytometry, which is more sensitive.12
To the best of our knowledge, this case is the first report of DLBCL with complete loss of CD19 expression at diagnosis, as demonstrated by flow cytometry, which is the most sensitive technique. This observation is explained by an acquired splice mutation in the CD19 gene, followed by an acquired uniparental disomy, leading to homozygous intron retention and a premature stop codon. Loss of expression of CD19 and other B-cell markers has been described in a case of high grade lymphoid neoplasm with features intermediate between DLBCL and acute lymphoblastic leukemia (TdT-positive) with a t(8;14) translocation involving cMYC.13 The emergence of subclones with splice mutations of CD19 has been reported in acute lymphoblastic leukemias treated with CAR-T cells targeting CD19, although the mutation referred to this report was never specified.14 In these cases, the CD19-negative subclones present a competitive advantage under the strong selective pressure exerted by the therapeutic intervention. It is therefore intriguing to observe this genetic inactivation in the absence of any therapeutic selective pressure.
The absence of CD19 expression in DLBCL is a diagnostic pitfall, because this antigen is widely used to gate the B cells for flow cytometry analysis15. More importantly, our observation suggests that it could be important in cases of DLBCL to evaluate the expression of CD19 when a targeted therapy (e.g., CAR-T cells) is considered, in order to detect those rare CD19-negative cases that are unlikely to benefit from therapies targeting CD19.
Supplementary Material
Acknowledgments
We would like to thank Philip Robinson (DRCI, Hospices Civils de Lyon) for help in manuscript preparation.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Wang K, Wei G, Liu D. CD19: a biomarker for B-cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Depoil D, Fleire S, Treanor BL, et al. CD19 is essential for B cell activation by promoting B cell receptor–antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9(1):63–72. [DOI] [PubMed] [Google Scholar]
- 3.He X, Kläsener K, Iype JM, et al. Continuous signaling of CD79b and CD19 is required for the fitness of Burkitt lymphoma B cells. EMBO J. 2018;37(11):e97980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung EY, Psathas JN, Yu D, Li Y, Weiss MJ, Thomas-Tikhonenko A. CD19 is a major B cell receptor–independent activator of MYC-driven B-lymphomagenesis. J Clin Invest. 2012;122(6):2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Zelm MC, Reisli I, van der Burg M, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354(18):1901–1912. [DOI] [PubMed] [Google Scholar]
- 6.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323–2330. [DOI] [PubMed] [Google Scholar]
- 7.Watkins MP, Bartlett NL. CD19-targeted immunotherapies for treatment of patients with non-Hodgkin B-cell lymphomas. Expert Opin Investig Drugs. 2018;27(7):601–611. [DOI] [PubMed] [Google Scholar]
- 8.Mareschal S, Ruminy P, Bagacean C, et al. Accurate classification of germinal center B-cell–like/activated B-cell–like diffuse large B-Cell lymphoma using a simple and rapid reverse transcriptase–multiplex ligation-dependent probe amplification assay. J Mol Diagn. 2015;17(3):273–283. [DOI] [PubMed] [Google Scholar]
- 9.Masir N, Marafioti T, Jones M, et al. Loss of CD19 expression in B-cell neoplasms. Histopathology. 2006;48(3):239–246. [DOI] [PubMed] [Google Scholar]
- 10.Nakatsuka S, Yutani C, Kurashige M, et al. An unusual case of Epstein-Barr virus-positive large B-cell lymphoma lacking various B-cell markers. Diagn Pathol. 2017;12(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazantseva M, Hung NA, Mehta S, et al. Tumor protein 53 mutations are enriched in diffuse large B-cell lymphoma with irregular CD19 marker expression. Sci Rep. 2017;7(1):1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, Agrawal N, Patel J, et al. Diminished expression of CD19 in B-cell lymphomas. Cytometry B Clin Cytom. 2005;63(1):28–35. [DOI] [PubMed] [Google Scholar]
- 13.Soliman DS, Al-Sabbagh A, Ibrahim F, et al. High grade B-cell Neoplasm with surface light chain restriction and Tdt coexpression evolved in a MYC-rearranged diffuse large B-Cell lymphoma: a dilemma in classification. Case Rep Hematol. 2017;2017:6891957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dongen JJ, Lhermitte L, Böttcher S, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.