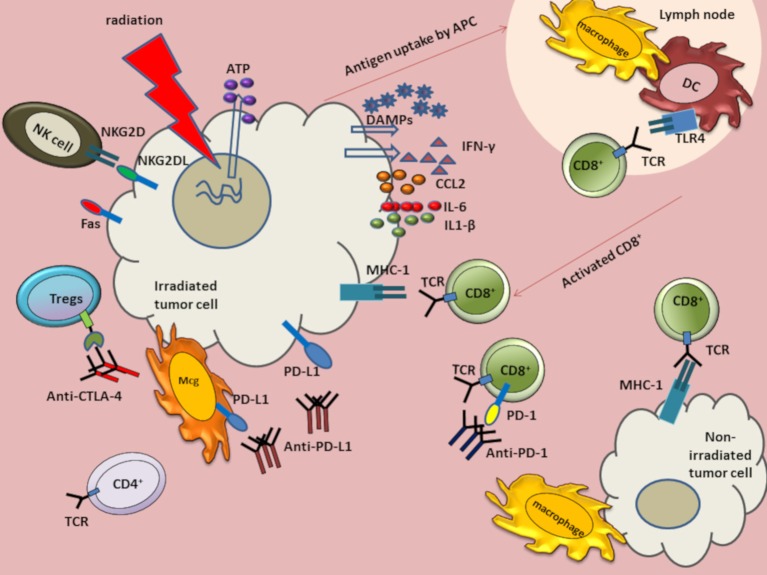
Figure 1.
Anti-tumor immune response augmented by the abscopal effect of radiation in combination with immunotherapies. Radiation induces DNA damage and cell death. The dying cells release ATP and DAMPs such as HMGB1 and calreticulin. Although HMGB1 binds TLR4, ATP and calreticulin modulate TLR4 signaling without directly binding to TLR4. Radiation also induces release of tumor antigens to antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs). Antigens are then processed and presented on major histocompatibility complex (MHC) Class I molecules to activate and induce proliferation of CD8+ T cells. The activated cytotoxic CD8+ T cells migrate to tumor sites to induce cell death. Radiation can also induce release of cytokines IL-6 and interferon-gamma (IFN-γ). Radiation also increases tumor cell expression of programmed cell death-1 ligand (PD-L1) and MHC class I molecules. Radiation upregulates immunomodulatory surface proteins, such as Fas and NKG2D ligands on tumor cells. The NKG2D upregulation facilitates NK-mediated tumor cell death. Antibodies, such as α-CTLA-4, α-PD-L1, and α-PD-1 have been used as cancer immunotherapies. When combined with radiation, these antibodies can augment anti-tumor responses in GBM. Anti-CTLA-4 can bind CTLA-4 on Tregs and downregulate suppressive activity. Anti-PDL1 can interact with PD-L1 on tumor cells and on myeloid derived suppressor cells (MDSCs) to curtail suppressive activity induced by MDSCs. Anti-PD-1 antibody can bind to programmed cell death-1 (PD-1) expressed on exhausted T cells.