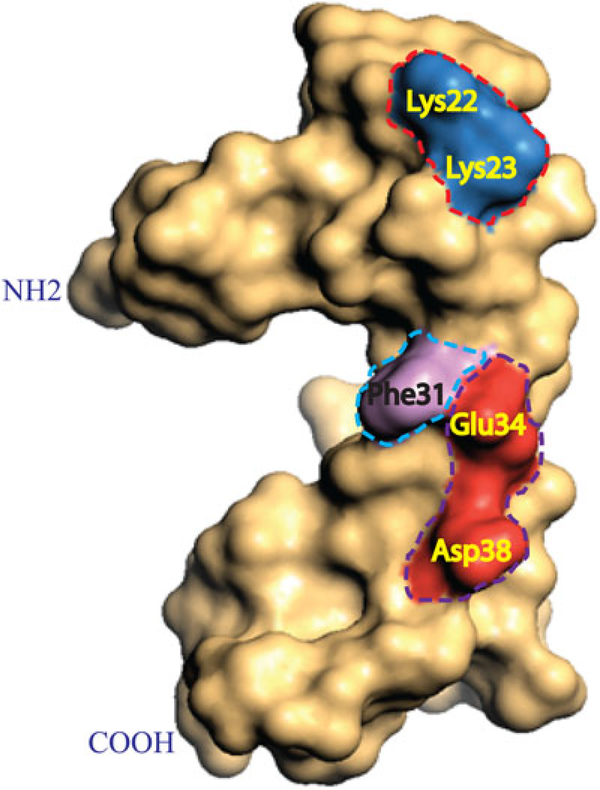
FIGURE 6.
Hydrophilic residues located on the major α‐helix domain play important roles in release of agnoprotein. Three-dimensional structure of the full‐length agnoprotein is illustrated in a “surface fill‐in” representation. The hydrophilic residues located on the hydrophilic face of the major α‐helix domain of agnoprotein, including Lys22, Lys23, Glu34, and Asp38; and an aromatic residue (Phe31), which is adjacent to this hydrophilic surface were determined to be critical for the release of agnoprotein from agnoprotein‐positive cells (Saribas, White, et al., 2018). Amino acid residues involved in agnoprotein release are encased in boxes and named. Note that Lys23, Lys23, Phe31, and Glu34 are all conversed between JCV, BKV, and SV40 agnoproteins. JCV Asp38 is substituted for Glu and Gln for BKV and SV40 agnoproteins, respectively, at the same position (Saribas, White, et al., 2018). BKV: BK virus; JCV: JC virus; SV40: simian virus 40 [Color figure can be viewed at wileyonlinelibrary.com]