Abstract
Debilitating perceptual disorders including tinnitus, hyperacusis, phantom limb pain and visual release hallucinations may reflect aberrant patterns of neural activity in central sensory pathways following a loss of peripheral sensory input. Here, we explore short- and long-term changes in gene expression that may contribute to hyperexcitability following a sudden, profound loss of auditory input to one ear. We used fluorescence in situ hybridization to quantify mRNA levels for genes encoding AMPA and GABAA receptor subunits (Gria2 and Gabra1, respectively) in single neurons from the inferior colliculus (IC) and auditory cortex (ACtx). Thirty days after unilateral hearing loss, Gria2 levels were significantly increased while Gabra1 levels were significantly decreased. Transcriptional rebalancing was more pronounced in ACtx than IC and bore no obvious relationship to the degree of hearing loss. By contrast to the opposing, synergistic shifts in Gria2 and Gabra1 observed 30 days after hearing loss, we found that transcription levels for both genes were equivalently reduced after 5 days of hearing loss, producing no net change in the excitatory/inhibitory transcriptional balance. Opposing transcriptional shifts in AMPA and GABA receptor genes that emerge several weeks after a peripheral insult could promote both sensitization and disinhibition to support a homeostatic recovery of neural activity following auditory deprivation. Imprecise transcriptional changes could also drive the system towards perceptual hypersensitivity, degraded temporal processing and the irrepressible perception of non-existent environmental stimuli, a trio of perceptual impairments that often accompany chronic sensory deprivation.
Keywords: Tinnitus, Hyperacusis, Hearing Loss, Auditory Neuropathy, Homeostatic Plasticity, Gene Transcription
Introduction
An acute loss of peripheral sensory input in adulthood triggers widespread compensatory changes in the central visual, auditory, and somatosensory pathways (Merzenich et al., 1983; Robertson and Irvine, 1989; Kaas et al., 1990; Jones and Pons, 1998; Wang et al., 2002; Kamke et al., 2003; Petrus et al., 2015; Humanes-Valera et al., 2017; Jaepel et al., 2017; Jiang et al., 2017; Asokan et al., 2018). For example, lesioning approximately 95% of cochlear nerve afferent synapses virtually eliminates sound-evoked responses in the auditory nerve, yet auditory responses recover nearly to baseline levels over a several week period in the auditory cortex (ACtx) (Chambers et al., 2016a; Resnik and Polley, 2017). Increased “central gain” in downstream areas of central auditory processing may support an adaptive recovery of sound detection thresholds despite widespread peripheral damage (Schuknecht and Woellner, 1953; Zeng, 2005; Lobarinas et al., 2013; Chambers et al., 2016a). The perceptual benefits of increased neural amplification are offset by a greater risk for debilitating perceptual consequences including hypersensitivity to moderately intense stimuli (e.g., hyperacusis) or the perceptual attribution of phantom stimuli to deafferented regions of the periphery (e.g., phantom limb pain, visual release hallucinations, or tinnitus) (Yang et al, 2007; Salvi et al, 2017).
If pathologically over-powered “neural amplifiers” in sensory brain areas are at the root of these perceptual disorders, developing strategies to turn down their gain will require a detailed understanding of the biological mechanisms supporting neural amplification. Activity-dependent shifts in neural activity partly arise through number, subunit composition or cellular distribution of neurotransmitter receptors (O’Brien et al., 1998; Kilman et al., 2002; Marsden et al., 2007; Zhang et al., 2015). Dynamic shifts in postsynaptic receptor expression accompany normal auditory learning (Sun et al. 2005; Cai et al 2010) and development (Caicedo and Eybalin 1999; Kotak et al 1998; Sanes and Kotak, 2011). Age-related hearing loss is accompanied by changes in GABA receptor distributions across the IC and ACtx (Gutiérrez et al., 1994; Milbrandt et al., 1994, 1997; Raza et al., 1994; Caspary et al., 1995, 2013; Yu et al., 2006), alongside changes in NMDA receptor distributions (Shim et al., 2012). Further, acute cochlear trauma leads to altered distributions of excitatory and inhibitory postsynaptic receptors in the brainstem (Suneja et al., 2000; Dong et al., 2009, 2010a), IC (Holt et al., 2005; Dong et al., 2010b), and ACtx (Wang et al., 2005).
In this report, we investigated changes transcription levels for genes encoding subunits of excitatory and inhibitory neurotransmitter receptors following a sudden loss of auditory peripheral input in young adult animals. Prior reports in several different sensory systems have described reduced GABAA receptor expression following a loss of peripheral afferent input (Wong-Riley and Jacobs, 2002; Kumar et al, 1994; Garraghty et al, 2006; Mowery et al, 2014), including the adult auditory system (Suneja et al., 2000; Dong et al., 2010b; Yang et al., 2011). Sensory neurons can also compensate for reduced activity levels through increased expression of glutamatergic AMPA receptors (Turrigiano et al., 1998; Suneja et al., 2000; Holt et al., 2005; Dong et al., 2010a; Teichert et al., 2017). We therefore chose to quantify mRNA levels of Gria2, which encodes the GluA2 subunit of AMPA receptors, and Gabra1, which encodes the α1 subunit of GABAA receptors, approximately one month following a near-complete loss of cochlear afferent neurons. Transcriptional shifts in Gria2 and Gabra1 expression were compared between the IC and ACtx, given documented differences the degree of compensatory plasticity between the midbrain and forebrain (Qiu et al., 2000; Chambers et al., 2016a). By taking advantage of novel fluorescence mRNA hybridization techniques that allow multi-channel single-molecule visualization in tissue sections, we quantified Gria2 and Gabra1 mRNA levels within single cells in each condition. Using this approach, we find opposing shifts in Gria2 and Gabra1 expression in the IC and ACtx after one month of near-complete cochlear denervation. Transcriptional shifts are proportionately larger in ACtx compared to the IC, which could underlie the more robust recovery of physiological responsiveness at the level of the cortex, when compared to the midbrain.
Experimental Procedures
All procedures were approved by the Institutional Animal Care and Use Committee at the Massachusetts Eye and Ear Infirmary and followed the guidelines established by the National Institutes of Health for the care and use of laboratory animals. Seven male CBA/CaJ mice (Jackson Labs), aged 10–12 weeks, and six CamKII-tTA × tetO-GCaMP6s mice on a C57BL6 background of both sexes (Wekselblatt et al., 2016), aged 5–6 weeks, were used in this study.
Unilateral cochlear denervation with ouabain
CBA/CaJ mice were anesthetized with ketamine (120mg/kg) and xylazine (12mg/kg), with supplemental doses of ketamine (60 mg/kg) administered as needed. Core body temperature was maintained at 36.7° C with a homeothermic heating pad. After numbing the left ear with a local anesthetic, a semicircular incision was made and the superficial fascia and muscle tissue were blunt retracted to expose the bulla. A small opening was made in the bulla with a 28.5-gauge needle to expose the round window niche. The exposed round window niche was then either filled with a ouabain solution (1–2 μL, 1 mM in distilled water; N = 4) or with distilled water vehicle (N = 3) using a blunted needle. Ouabain or distilled water was reapplied 5–7 more times at 15-minute intervals, wicking the existing solution away before each new application. Distortion product otoacoustic emission (DPOAE) and auditory brainstem response (ABR) were measured following the sixth application, either to confirm normal DPOAE and ABR thresholds in vehicle-treated mice or elevated ABR thresholds with normal DPOAE in ouabain-treated mice. If ABR thresholds with 16 kHz tone pips (see below) were < 60 dB SPL after six ouabain applications, one or two more applications were performed until the ABR threshold was > 60 dB SPL without inducing any obvious shift in DPOAE threshold. The incision was closed, Bacitracin applied to the wound margin, and Buprenex (0.5mg/kg) administered subcutaneously as an analgesic. Mice were transferred to a heated recovery chamber before returning to their home cage.
Unilateral cochlear deafening with sterile water
Short-term unilateral deafening (STUD) or a control procedure was performed on a separate cohort of transgenic mice (N=6) on a C57BL6 background (CamKII-tTA × tetO-GCaMP6s); Jackson labs stock numbers 003010 and 024742, respectively). Mice were brought to a surgical plane of anesthesia using ketamine (120mg/kg) and xylazine (12mg/kg), with supplemental doses of ketamine (60 mg/kg) administered as needed. Before inducing STUD (N = 3 mice), DPOAEs and ABRs were first measured in the left ear to confirm normal cochlear function. The tympanic membrane and middle ear ossicles were then removed with fine forceps. A flexible canula was then attached to the exposed oval window and a1–2μL bolus of distilled water was flushed through the oval window opening. ABR and DPOAE were measured after every 2–4 flushes until the DPOAE was not measurable and the ABR threshold was > 60 dB SPL. The middle ear cavity was then packed with sterile cotton, the pinna incision was sutured closed and covered with Bacitracin. Buprenex (0.5mg/kg) was administered subcutaneously before mice were transferred to a warmed recovery chamber. For the control procedure (N = 3), DPOAEs and ABRs were measured in the left ear, Buprenex (0.5mg/kg) was subcutaneously administered, and animals were transferred to a heated chamber to recover as described above. Five days after control or STUD surgery, animals were anesthetized as described above and sterile cotton removed from the middle ear. DPOAE and ABR thresholds were measured in the left ear to confirm profound elevations in ABR and DPOAE thresholds in STUD mice and normal thresholds in control mice.
Cochlear function tests
ABR and DPOAEs were measured at a single frequency (16 kHz) during the cochlear denervation surgery and measured again at multiple frequencies just prior to processing of brain tissue (Figure 1A). All measurements were performed under anesthesia with core body temperature maintained at 36.7° C, as described above. ABR recordings were made with transdermal electrodes (Grass Technologies, Natus Medical Inc.) either arranged in the standard pinna to vertex montage (ouabain denervation) or a pinna-pinna horizontal montage (STUD) and focused on wave 1, which 2006). Acoustic stimuli were generated with a 24-bit digital-to-analog converter (PXI-4461, National Instruments) and delivered using custom in-ear acoustic assemblies consisting of two miniature dynamic earphones (CUI CDMG15008–03A) and an electret condenser microphone (Knowles FG-23339-PO7) coupled to a probe tube. Sound levels were calibrated in the ear canal for each mouse prior to every recording session.
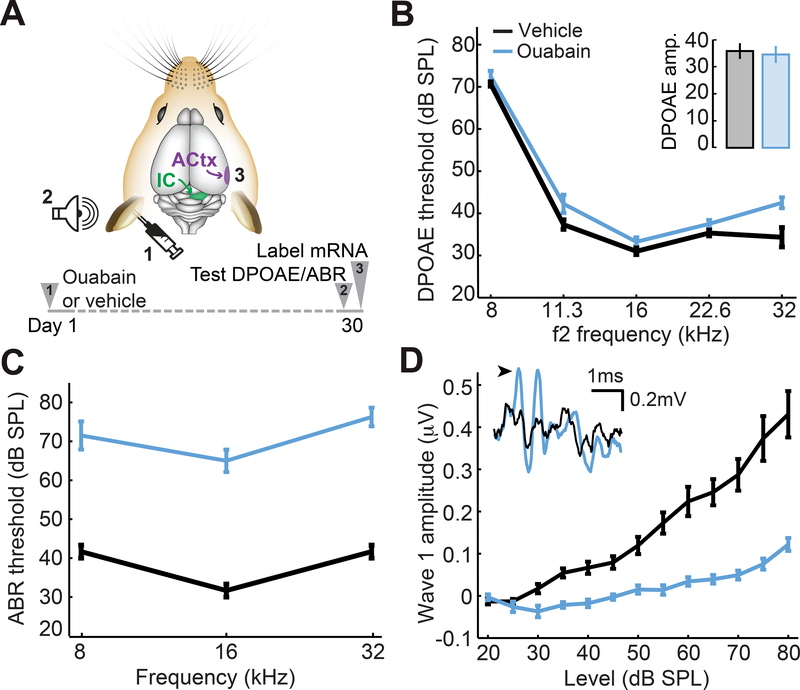
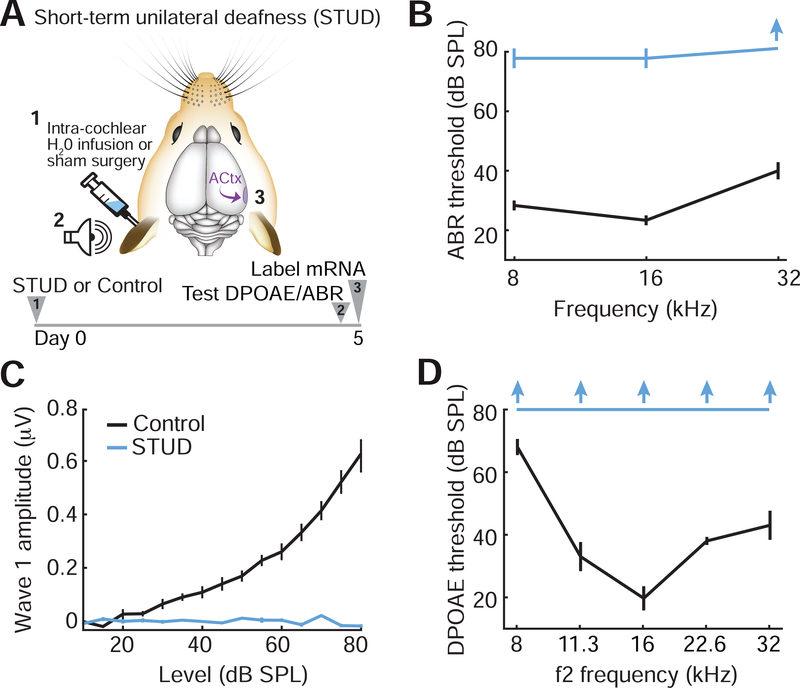
Figure 1. Cochlear application of ouabain elevates ABR thresholds with minimal effects on DPOAEs.
(A) A 1mM Ouabain solution or distilled water (vehicle) was applied to the left ear of adult CBA/CaJ mice. Distortion product otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABRs) were measured 30 days later, just before processing tissue from the contralateral inferior colliculus (IC) and auditory cortex (ACtx) for in situ hybridization. (B) DPOAE thresholds from ouabain- and vehicle-treated ears (blue and black, respectively). Inset: DPOAE emission amplitude at 60 dB SPL from the same ears. (C-D) ABR wave 1b thresholds presented at particular test frequencies (C) or wave 1b amplitude growth functions averaged across test frequencies (D). Inset: representative ABR waveforms from vehicle- and ouabain-treated ears evoked by 16kHz tone pips at 70dB SPL. Black arrowhead denotes wave 1b. All data are mean ± SEM.
DPOAE stimuli were primary tones – 8, 11.3, 16, 22.6, and 32kHz – presented in 5dB steps from 20 to 80dB SPL, with a frequency ratio of 1.2 and the f2 primary level 10dB below the corresponding f1 level. We then calculated the sound pressure level at the 2f1-f2 DPOAE frequency as well as the acoustic noise floor. DPOAE threshold was defined as the lower of at least two continuous levels where the DPOAE was at least 5dB above the acoustic noise floor. The ABR was elicited with tone pips (8, 16, and 32kHz, 5ms duration, with 0.5ms raised cosine onset and offset ramps). Tones were presented in 5dB steps from 20 to 80dB SPL, repeated 512 times each. ABR threshold was defined at each frequency as the lowest sound level at which a repeatable waveform could be identified. Visual identification of the waveform was validated with a semi-automated algorithm that identifies peaks and troughs of putative ABR waves by first calculating the negative zero crossings of the first derivative of the recorded waveform. The algorithm eliminates spurious peaks by setting a threshold for negative zero crossing amplitude based on the noise floor, calculated from the standard deviation of the first 1 ms of the signal (Buran et al., 2010).
Tissue Acquisition and in situ hybridization
Unfixed brains were extracted and flash frozen in liquid nitrogen, and then embedded in OCT compound (TissueTek, VWR, Radnor, PA). Embedded brains were secured in a −28°C cryostat and cut into 10μm coronal sections. Frozen sections were mounted on pre-chilled (−20°C) Superfrost slides (Fisher Scientific, Waltham MA) and stored at −80°C. Fluorescence in situ hybridization for Gria2 and Gabra1 mRNA was performed on sections containing the ACtx or IC. Custom target probes were provided by Advanced Cell Diagnostics (ACD, Hayward CA, USA), described previously (Hackett et al., 2015). Tissue permeabilization, mRNA hybridization and amplification, and fluorescent labeling were all performed using the RNAscope Multiplex Fluorescent Reagent Kit and HyBEZ oven (ACD, Hayward CA, USA), according to the manufacturer’s instructions for fresh-frozen brain tissue. Cell nuclei were counterstained with DAPI and sections were coverslipped with Vectashield (Vector Labs, Burlingame CA).
Image Acquisition and analysis
For cochlear denervation experiments, three regions of interest (185 μm x 185 μm each) were imaged at three distinct caudal-rostral positions within the IC and ACtx. In the IC, all regions of interest were positioned centrally, in and around the central nucleus. In the ACtx, all regions of interest were positioned between layer 2/3 and layer 6 along a single cortical column. Anatomical landmarks were cross-referenced against our prior publications and published mouse brain atlases to ensure that all cortical ROIs fell inside the boundaries of ACtx (Hackett et al., 2011, 2015). Images were acquired with a Leica SP8 confocal microscope with a 63× 0.8NA immersion lens and two Leica HyD detectors. Image stacks were transferred to image-processing software (Amira, Visage Imaging) and projected in three dimensions. Perimeters of DAPI-labeled nuclei were identified by vectors of maximal intensity contrast between the DAPI-labeled region and image background, which consistently demarcated the outer edge of the labeled nucleus. Neighboring DAPI nuclei that were not segregated based on intensity contrast were manually separated using the Volume Edit tool in Amira. Large clusters of overlapping nuclei that could not be manually separated were excluded from analysis. Partial segments of DAPI nuclei, located along the image border or above and below the imaging planes, were also excluded from analysis. In the Gria2 and Gabra1 fluorophore channels, clusters of fluorescent pixels with a minimum diameter of 0.7–0.9 μm (4–5 pixels) were operationally classified as individual Gria2 or Gabra1 puncta. Single fluorescent clusters correspond to single mRNA copies, as previously described (Wang et al., 2012). Large pixel clusters that could be visually separated into individual puncta were also subdivided using the Volume Edit tool in Amira; clusters that exceeded 1.5 μm (8–10 pixels) and could not be visually separated were excluded from analysis. Identified Gria2 and Gabra1 puncta within 2.5–3.5μm (15 to 20 pixels) of a DAPI-labeled perimeter were then counted and assigned to that cell using the Connected Components function in Amira. Transcript counts per cell were then exported to MATLAB (Mathworks).
Analysis of tissue from mice that underwent the STUD protocol focused only on ACtx, where three columnar regions across the caudal-rostral extent were imaged at 63× using a Leica DM5500B fluorescent microscope. Image stacks within each ROI were captured in 210 μm x 210 μm x 10 μm stacks with 0.5μm z-plane spacing and stitched together using the Mosaic Merge tool in the Leica scope software (LASX). Tiled image stacks were then separated into individual fluorophore channels, and images were deconvolved in the Z dimension using the 3D deconvolution tool in LASX. Images for each channel were collapsed across the z plane using the Maximum Intensity Projection tool in LASX, and then exported to MATLAB for further analysis. In MATLAB, images were converted to binary using a 50% threshold, which removed background fluorescence while preserving DAPI and mRNA label.
In DAPI images, individual cells were identified as isolated pixel clusters within 5–15 μm. In mRNA images, fluorescent puncta corresponding to individual mRNA transcripts were identified as isolated pixel clusters with diameters between 0.7–0.9μm. Smaller clusters in all images were excluded from analysis. Larger clusters were further segmented by computing a distance transform within each cluster, generating a grayscale intensity image based on the distance transform, and applying a watershed algorithm to the grayscale image. Segmented regions were then identified as isolated cells or puncta based on the size requirements described above. Perimeters of identified DAPI nuclei were then radially dilated to delineate a putative cytosolic region for each cell, and isolated fluorescent puncta within this region in each fluorophore channel were counted and assigned to that cell. If dilated perimeters overlapped between cells, they were discarded from analysis. Cells with actual or dilated perimeters contacting the edge of the image were also discarded from analysis.
Statistics
Statistical analysis was performed in Matlab. The Lillefors test was used to determine whether any given sample was normally distributed. If data met the assumptions of parametric statistics, descriptive statistics were provided as means with standard errors and inferential statistics were performed with a mixed model ANOVA or unpaired t-tests. If data did not meet the assumptions of parametric statistics, descriptive statistics were provided as the median of a distribution and inferential statistics were performed with the Wilcoxon Rank Sum test.
Results
Unilateral auditory nerve damage following application of ouabain to the cochlear round window
We selectively lesioned primary afferent neurons throughout the cochlear frequency map by applying ouabain to the cochlear round window. Ouabain is a Na2+/K+ ATP-ase pump inhibitor that eliminates Type-I cochlear afferent neurons while inducing little damage to other sensory and nonsensory cell types in the inner ear (Lang et al., 2005; Yuan et al., 2013). In keeping with previous reports, we found that repeated application of ouabain at 1mM concentration to the left ear was associated with a slight elevation of distortion product otoacoustic emission (DPOAE) threshold, a marker of pre-neural outer hair cell function (Fig. 1B, ANOVA, F(1)=12.2, p < 0.05). Other than this modest threshold shift, hair cell function appeared normal, as DPOAE amplitudes measured at a suprathreshold level (60 dB SPL) were not different between ouabain- and vehicle-treated mice (Fig. 1B inset, unpaired t-test, p = 0.77).
By contrast, sound-evoked responses in the auditory nerve and brainstem were profoundly reduced due to the loss of primary afferent neurons that convey auditory signals from the inner ear to the brain. Thirty days following ouabain treatment, auditory brainstem response (ABR) thresholds were elevated by 30–40 dB across all test frequencies compared to vehicle-operated controls (Fig. 1C, ANOVA, F(1)=90.9, p < 0.0005). Wave 1 of the ABR is generated by the synchronized compound action potentials of Type-I spiral ganglion neurons, where the amplitude of wave 1 is linearly related to the fraction of surviving synapses onto inner hair cells (Yuan et al., 2013; Chambers et al., 2016a). We found that the wave 1 sound level growth function was reduced by approximately 75% compared to vehicle-treated controls (Fig. 1D, ANOVA, F(1)=48.2, p < 0.0001).
Opposing transcriptional shifts in AMPA and GABAA receptor subunit mRNA following auditory nerve damage
With a protocol in place to reduce afferent input from the auditory nerve, we next developed a strategy to quantify transcriptional changes in central auditory neurons that may support a compensatory plasticity. Given the long-recognized linkage between auditory deprivation and changes in GABA and AMPA receptors following auditory deprivation, we focused on quantifying changes in Gria2 and Gabra1 mRNA, which encode the GluA2 and α1 subunits of AMPA and GABAA receptors, respectively. We focused our analysis on the ACtx and IC contralateral to the denervated ear based on prior comparisons of physiological plasticity in these brain areas following selective cochlear afferent damage (Qiu et al., 2000; Chambers et al., 2016a).
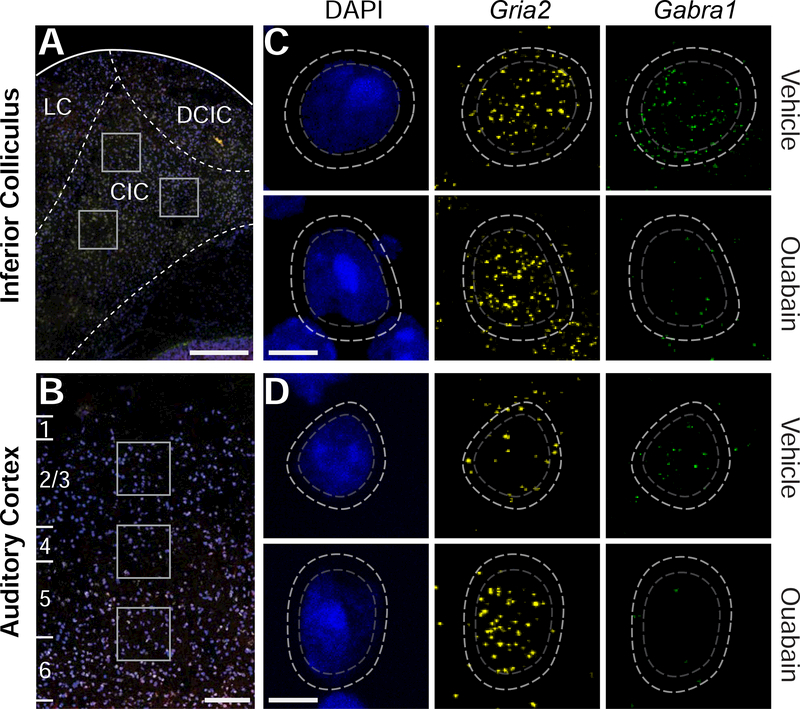
We used fluorescent in situ hybridization followed by quantitative image analysis to label and count mRNA transcripts encoding AMPA and GABAA receptor subunits in single cells 30 days after ouabain (N = 4 mice) or vehicle (N = 3 mice) treatment. Gria2 and Gabra1 mRNA transcripts were labeled in the peri-nuclear area of individual neurons visualized in coronal sections through IC (Fig. 2A) and ACtx (Fig. 2B). As shown in these four representative neurons, we observed that Gria2 and Gabra1 transcript counts were approximately balanced in the IC and ACtx of vehicle-treated mice, but shifted in opposing directions 30 days after contralateral denervation, such that Gria2 mRNA levels were increased while Gabra1 levels were reduced (Fig. 2C-D, top vs bottom rows, respectively).
Figure 2. Quantification of Gria2 and Gabra1 mRNA transcripts from single neurons in the inferior colliculus and auditory cortex.
(A-B) Individual mRNA transcripts that encode subunits of AMPA and GABAA receptors (Gria2 and Gabra1, respectively) were measured from regions of interest (white squares) in the IC (A) or ACtx (B) contralateral to the vehicle- or ouabain-treated ear. LC, DCIC and CIC represent the approximate boundaries of the Lateral Cortex, Dorsal Cortex of the IC and Central Nucleus of the IC, respectively. (C-D) Single cells were identified by DAPI labeling of the nuclear perimeter (dashed gray line). Representative Gria2 (yellow) and Gabra1 (green) mRNA levels in individual cells contralateral to vehicle- and ouabain-treated ears (top and bottom row, respectively). Fluorescently labeled individual mRNA transcripts are automatically identified within a fixed radius of each nucleus (dashed white line), counted, and then assigned to a given cell. Scale bars in A and B are 250 μm. Scale bars in C and D are 5μm.
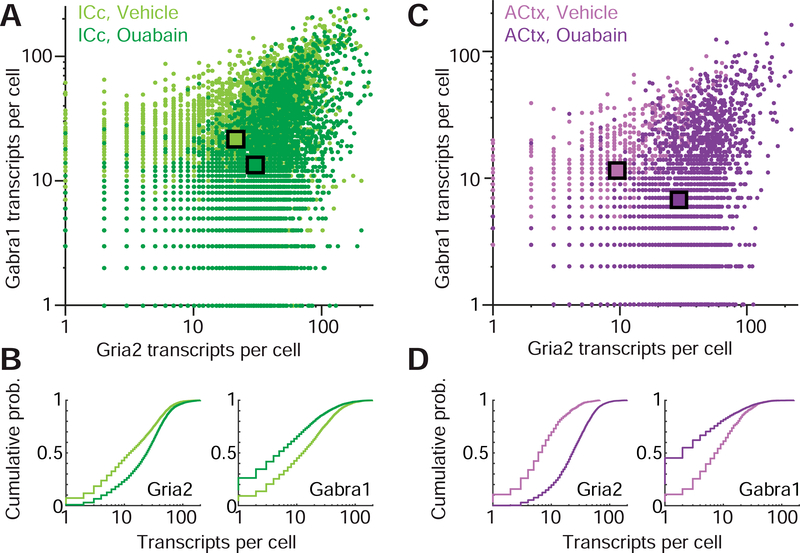
To quantify these changes, we implemented an automated analysis routine that i) identified single mRNA puncta, ii) assigned them to a parent neuron, and iii) counted them to quantify Gria2 and Gabra1 levels in individual neurons. Using this approach, we were able to quantify transcriptional markers for both excitatory and inhibitory neurotransmission in thousands of single neurons in the IC (vehicle-treated, n= 4125 cells, ouabain-treated, n = 6063 cells) and ACtx (vehicle, n = 2767 cells, ouabain, n = 4705 cells). In the IC, we observed that contralateral cochlear denervation significantly increased Gria2 levels compared to vehicle controls (30.54 ± 0.36 vs. 21.86 ± 0.29, mean ± SEM for ouabain and vehicle, respectively, unpaired t-test, p < 1 × 10−6). In the same cells, we observed that Gabra1 transcripts were significantly reduced (13.34 ± 0.28 vs. 23.28 ± 0.32, mean ± SEM for ouabain and vehicle, respectively, unpaired t-test, p < 1 × 10−6, Fig. 3A-B). Opposing shifts in mRNA levels were also seen in ACtx, where cells expressed significantly higher levels of Gria2 transcripts than controls (28.98 ± 0.34 vs. 9.52 ± 0.45, mean ± SEM for ouabain and vehicle, respectively, unpaired t-test, p < 1 × 106) but significantly lower levels of Gabra1 mRNA (6.73 ± 0.34 vs. 11.85 ± 0.53, mean ± SEM for ouabain and vehicle, respectively, unpaired t-test, p < 1 × 106, Fig. 3C-D).
Figure 3. Synergistic shifts in Gria2 and Gabra1 transcription following cochlear nerve damage.
(A) Counts of Gria2 and Gabra1 transcripts in the IC contralateral to the vehicle- and ouabain-treated ears (light and dark green hue, respectively). Each point represents the number of transcripts for a given cell. Squares indicate means of the Gria2 and Gabra1 distributions for each treatment group. (B) Cumulative distributions of Gria2 and Gabra1 transcripts in vehicle- and ouabain-treated mice. (C-D) As per A and B, but for ACtx. Lighter and darker hues of purple represent cells contralateral to vehicle- and ouabain-treated ears, respectively.
Hierarchical regulation of excitatory/inhibitory balance
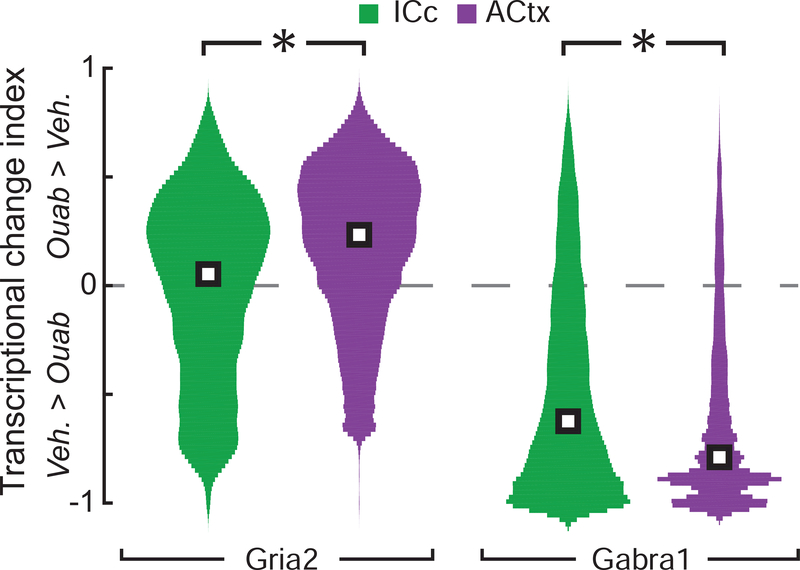
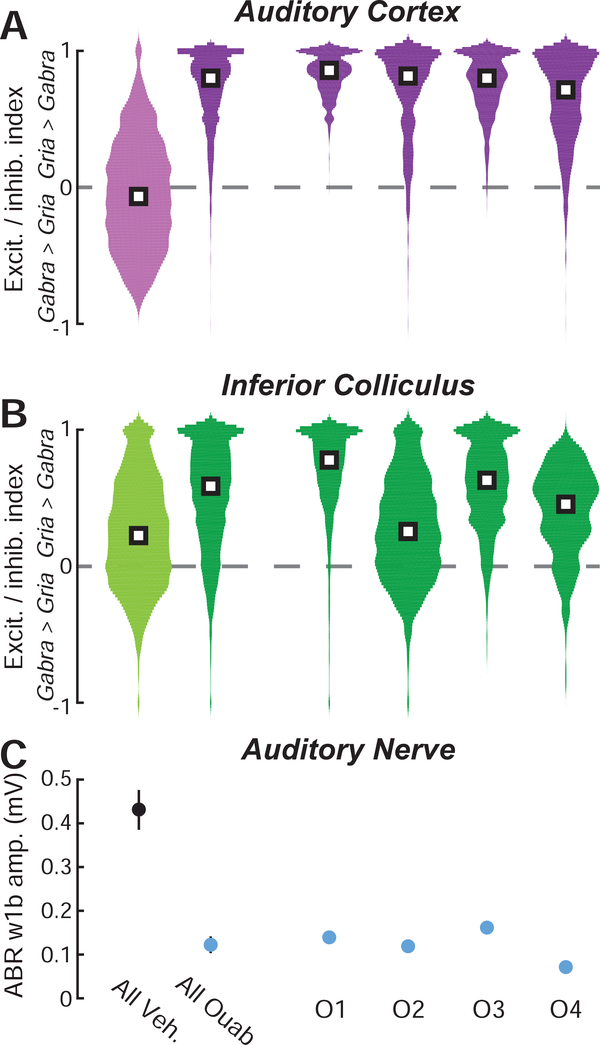
Prior reports have described more complete compensatory plasticity in ACtx than IC following ouabain denervation. To extend this analysis to transcriptional changes we computed an asymmetry index bounded from −1 to +1, where a value of 0 is equivalent to the mean of the vehicle distribution for Gria2 or Gabra1 from the corresponding brain region (Fig. 4). We observed that Gria2 elevation following contralateral denervation was more pronounced in the ACtx than in the IC (0.23 ± 0.51 vs. 0.05 ± 0.64, median ± IQR for ACtx and IC, respectively, Wilcoxon rank sum, p < 1 × 10−6). We also found that reductions in Gabra1 transcripts were significantly more pronounced in the ACtx compared to the IC (−0.79 ± 0.47 vs. −0.62 ± 0.76, median ± IQR for ACtx and IC, respectively, Wilcoxon rank sum, p < 1 × 10−6).
Figure 4. Bi-directional changes in Gria2 and Gabra1 transcripts are more pronounced in ACtx than IC.
Changes in Gria2 and Gabra1 transcript levels in each individual cell are expressed relative to the mean of the corresponding vehicle value according to the formula (# transcripts in single ouabain cell – mean transcript count from vehicle) / (# transcripts in single ouabain cell + mean transcript count from vehicle), where zero (dashed gray line) indicates no change relative to vehicle and positive and negative values represent increases or decreases relative to vehicle, respectively. White squares represent the median of each distribution. Veh. = vehicle. Asterisks indicate significant differences with a Wilcoxon Rank Sum test.
These findings suggest that central compensation for near-complete cochlear denervation could be supported, in part, through a synergistic increase in AMPA receptor availability (i.e., sensitization) and reduction in GABAA receptor availability (i.e., disinhibition). Together, sensitization and disinhibition would cooperatively tip the excitatory/inhibitory (E/I) balance in the central pathway towards hyperexcitability. We computed a composite excitation/inhibition (E/I) index in each cell according to the formula (Gria2 - Gabra1) / (Gria2 + Gabra1), where again a value of 0 indicates a balanced transcription of both genes, while positive and negative values indicate transcriptional changes consistent with hyper- or hypo-excitability, respectively. As expected from the results above, E/I indices were significantly more positive in both the ACtx (Fig. 5A) and IC (Fig. 5B) of ouabaintreated mice than vehicle-treated mice (Wilcoxon rank sum test, p < 1 × 10−6 for both).
Figure 5. Net transcriptional shifts show no obvious relationship to the extent of auditory nerve damage.
(A-B) The distribution of excitation/inhibition (E/I) index values from all cells in ACtx (A) and IC (B), according to the formula (Gria2 – Gabra1) / (Gria2 + Gabra1), where zero (dashed gray line) indicates an equivalent number of excitatory (Gria2) and inhibitory (Gabra1) transcripts. E/I index values are pooled across all vehicle and ouabain-treated mice (left) but are also shown separately for individual ouabain-treated mice (O1–4). (C) The mean amplitude of ABR wave1b at 80 dB SPL provides an index of auditory nerve damage in each ouabaintreated mouse (blue) as compared to vehicle-treated mice (black). Veh. = vehicle.
We sought to determine whether a consistent relationship existed between the degree of auditory nerve damage and the degree of rebalancing toward a more positive E/I index. We compared ABR Wave 1b amplitudes to the distribution of E/I indices from IC and ACtx in each ouabain-treated mouse (Fig. 5A-C, right, rank-ordered by cortical E/I index) but did not find any obvious relationship between the extent of peripheral neuropathy and the extent of transcriptional E/I balance in the IC or ACtx. For example, the ouabain-treated mice with the strongest and weakest ABR wave 1 amplitudes (O3 and O4 respectively) showed the least pronounced E/I shifts in ACtx and middling E/I shifts in the IC. Although the sample size here is too limited to make any strong conclusions, this confirms prior observations that the extent of compensatory plasticity is not strictly determined by the status of the sensory periphery, and instead may be more directly regulated by local circuit dynamics (Chambers et al., 2016b, 2016a; Resnik and Polley, 2017).
No synergistic shifts in AMPA and GABAA mRNA transcription levels following a shorter period of unilateral auditory deprivation
Neural recovery of sound processing following widespread but selective cochlear afferent loss takes at least one week to emerge and several weeks to reach maximum levels of compensation (Qiu et al., 2000; Chambers et al., 2016a; Resnik and Polley, 2017). Transcriptional changes that underlie homeostatic plasticity processes such as synaptic scaling are generally much faster, ramping up within hours following activity perturbations (Ibata et al., 2008). To assess whether opposing changes in Gria2 and Gabra1 mRNA levels following cochlear deafferentation could be an early marker of compensatory plasticity that would appear before physiological indices of excess central gain, we performed a follow-up study that measured transcriptional changes 5 days following unilateral cochlear deafferentation. We chose not to use ouabain for shorter term analyses because the degree of cochlear afferent neuropathy is a moving target that steadily increases throughout the first few weeks (Yuan et al., 2013).
To avoid the complications of interpreting transcriptional changes when cochlear afferent loss is not yet complete, we instead used a protocol that induced a more immediate unilateral sensorineural and conductive hearing loss. Immediate short-term unilateral deafness (STUD) was accomplished by removing the tympanic membrane and ossicles in the left ear, puncturing the cochlear oval window, infusing sterile water into the cochlea through the oval window and packing the middle ear space with an absorbent material to wick out cochlear fluids by capillary action (Fig. 6A). Stability of STUD was assessed by measuring cochlear functions 5 days later with the wick removed and the middle ear free of all fluid. When compared to control measurements, we observed that ABR thresholds in the STUD ear were elevated to or above the highest sound level tested (Fig. 6B), ABR wave 1 growth functions were flat (Fig. 6C) and DPOAE thresholds were not measurable at any frequency or level tested (Fig. 6D).
Figure 6. Cochlear sterile water perfusion induces complete short-term unilateral deafness.
(A) Schematic illustrates a protocol to induce short-term unilateral deafness (STUD) in the left ear through intracochlear perfusion of sterile water through the exposed cochlear oval window. Cochlear function and ACtx mRNA was measured 5 days after induction of STUD. (B-D) ABR threshold (B), wave 1 sound level growth functions (C) and DPOAE threshold (D) were measured in the left ear of control (black) or STUD (blue) mice. Upward arrows indicate no measurable response was detected at the highest sound level tested. All data are mean ± SEM.
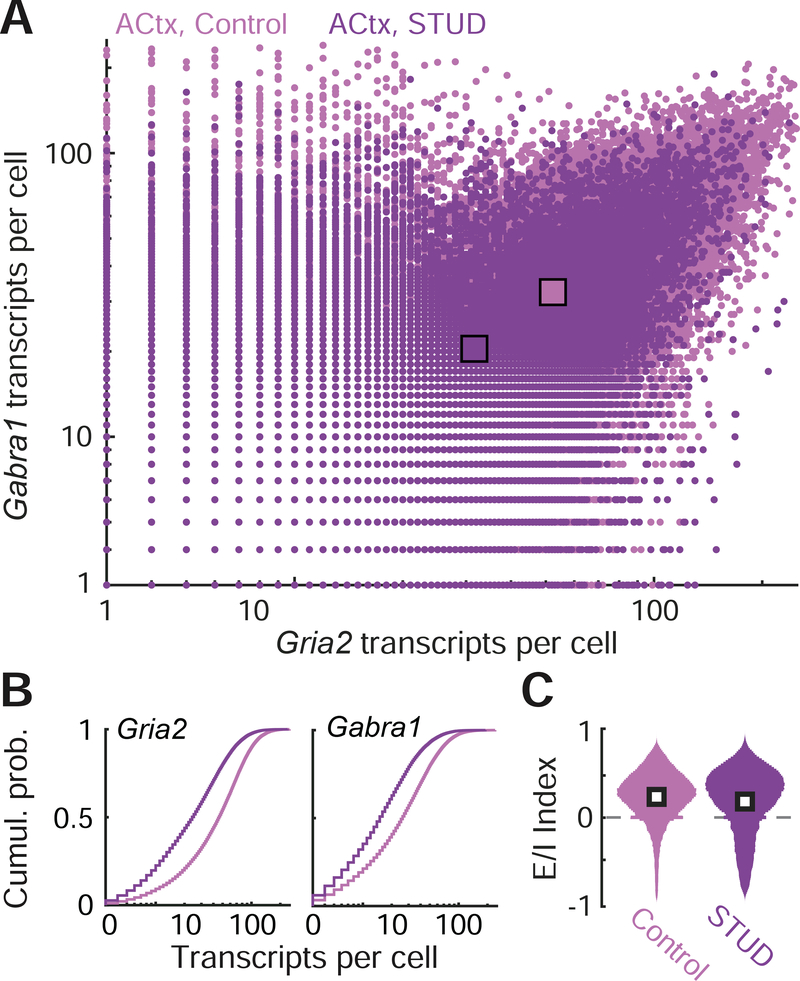
Gabra1 and Gria2 mRNA levels were quantified in individual neurons from the right ACtx 5 days after STUD (n = 13,282 from STUD and n = 19,862 from controls; Fig. 7A). We observed that Gabra1 levels were significantly reduced in STUD mice compared to controls (20.27 ± 0.17 vs. 37.2 ± 0.2, mean ± SEM for STUD and vehicle, respectively, unpaired t-test, p < 1 × 10−6), but Gria2 levels were also significantly lower (31.4 ± 0.23 vs. 58.9 ± 0.25, mean ± SEM for STUD and vehicle, respectively, unpaired t-test, p < 1 × 10−6; Fig. 7B). The reduction in Gabra1 and Gria2 transcription levels was approximately equivalent, leading to no clear change in the E/I index in STUD neurons compared to control (0.33 ± 0.58 vs. 0.30 ± 0.37, median ± IQR for STUD and vehicle, respectively; Fig. 7C).
Figure 7. Equivalent reduction in Gabra1 and Gria2 mRNA transcripts produce no net shift in excitatoryinhibitory balance following short-term unilateral deafness.
(A) Counts of Gria2 and Gabra1 transcripts in the ACtx contralateral to the deafened ear (dark purple hue) or in control mice (light purple hue). Each point represents the number of transcripts for a given cell. Squares indicate means of the Gria2 and Gabra1 distributions for each treatment group. (B) Cumulative distributions of Gria2 and Gabra1 transcripts in STUD and control mice. (C) The distribution of excitation/inhibition (E/I) index values from all cells in ACtx, according to the formula (Gria2 – Gabra1) / (Gria2 + Gabra1), where zero (dashed gray line) indicates an equivalent number of excitatory (Gria2) and inhibitory (Gabra1) transcripts. White squares represent the median of each distribution.
Downregulation of AMPA and GABAA mRNA transcription after STUD in genotyped excitatory and inhibitory neurons
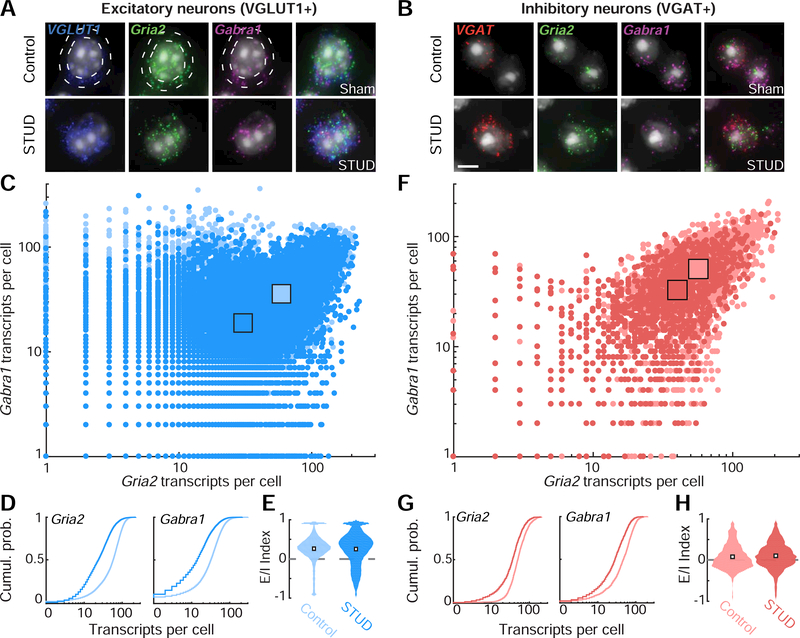
We questioned whether a cell type-specific mRNA analysis might reveal more subtle transcriptional rebalancing that was differentially expressed in excitatory and inhibitory neurons (Sturm et al., 2017). For example, excitatory neurons might predominantly express the decrease in Gabra1 (i.e., disinhibition), whereas inhibitory neurons could dominate the decreased expression of Gria2 (i.e., sensitization). This would produce a transcriptional rebalancing towards network hyperexcitability that could not be appreciated if all cell types were pooled together in a single analysis. We addressed this possibility by performing the same analysis of Gria2 and Gabra1 mRNA, in ACtx neurons that were genotyped as either excitatory or inhibitory, depending on whether they expressed vesicular glutamate transporter 1 (VGLUT1) or vesicular GABA transporter (VGAT) mRNA, respectively (Fig. 8A-B). Contrary to our prediction, we found that the commensurate downward regulation of both transcripts at the level of the population (Fig. 7) was supported by parallel changes in both excitatory neurons (Fig. 8C-E) and inhibitory neurons (Fig. 8F-H). Thus, in contrast to longer-term deafferentation following ouabain, we only found evidence for a matched reduction in both Gria2 and Gabra1 mRNA in ACtx neurons that produce no net change in the E/I balance in either excitatory or inhibitory ACtx neurons following shorter recovery periods.
Figure 8. Parallel reductions in Gabra1 and Gria2 mRNA levels after STUD are observed both in excitatory and inhibitory ACtx neurons.
(A-B) four-channel fluorescence microscopy supports identification of DAPIlabeled nuclei (white) alongside mRNA transcripts for Gria2 (green), Gabra1 (magenta) and either the vesicular glutate transporter 1 mRNA (VGLUT1, blue, A) or the vesicular GABA transporter (VGAT, red, B). The presence of VGLUT1 or VGAT mRNA in the perinuclear region (white circles in A) was used to genotype the neuron as excitatory or inhibitory and also to count the number of Gria2 and Gabra1 transcripts, as per all prior measurements. Scale bar = 5 μm. (C) Counts of Gria2 and Gabra1 transcripts in VGLUT1+ excitatory neurons in the ACtx contralateral to the deafened ear (dark blue hue) or in control mice (light blue hue). Each point represents the number of transcripts for a given cell. Squares indicate means of the Gria2 and Gabra1 distributions for each treatment group. (D) Cumulative distributions of Gria2 and Gabra1 transcripts from VGLUT1+ excitatory neurons in STUD and control mice. (E) The distribution of excitation/inhibition (E/I) index values from all VGLUT1+ excitatory cells in ACtx, according to the formula (Gria2 – Gabra1) / (Gria2 + Gabra1), where zero (dashed gray line) indicates an equivalent number of excitatory (Gria2) and inhibitory (Gabra1) transcripts. White squares represent the median of each distribution. (F-H) Same as C-E, but for VGAT+ inhibitory neurons.
Discussion
In this study, we reaffirmed that ouabain application to the cochlear round window membrane eliminates sound-evoked auditory brainstem responses while largely sparing pre-neural cochlear mechanics (Figure 1). We demonstrated that individual cells in the IC and ACtx increase Gria2 expression and decrease Gabra1 expression after 30 days of unilateral cochlear deafferentation (Figures 2 and 3). Both elevations in Gria2 levels and reductions in Gabra1 levels were more pronounced in the ACtx compared to the IC (Figure 4) but are not directly correlated with the extent of peripheral cochlear neuropathy (Figure 5). Synergistic shifts in Gria2 and Gabra1 expression were not observed following a shorter recovery period from unilateral hearing loss, where transcription levels of both genes were reduced (Figure 6–8).
Direct comparisons between the ouabain-based long-term deprivation and the shorter-term deprivation with STUD should be interpreted cautiously in light of procedural differences between the two experiments. For instance, different mouse strains were used for each method of hearing loss studies to say nothing of significant differences in the degree and form of unilateral hearing loss between the two approaches. Given that hearing loss was more complete with cochlear water infusion, this difference would have biased us towards finding a greater degree of compensatory changes following STUD compared with ouabain, not less. Instead, the findings reported here match prior physiological characterizations of central auditory recovery following cochlear afferent denervation, where enhanced central gain was more robust one month after afferent damage than one week and is also more pronounced in the thalamus and cortex than in the midbrain (Qiu et al., 2000; Chambers et al., 2016a, 2016b; Resnik and Polley, 2017).
Complementary changes in AMPA and GABAA subunit transcription within individual cells could reflect homeostatic plasticity mechanisms that scale postsynaptic receptor distributions in response to changes in afferent drive (Turrigiano, 2011). Activity perturbations in cultured neurons demonstrate that elevated Gria2 expression and subsequent AMPA receptor accumulation are critical steps in upward scaling of excitatory synaptic strength (O’Brien et al., 1998; Wierenga et al., 2005; Gainey et al., 2009; Lambo and Turrigiano, 2013), while reductions in GABAA receptor distributions lead to decreases in inhibitory synaptic strength (Kilman et al., 2002). Removing peripheral visual or somatosensory input in vivo can lead to opposing shifts in AMPA and GABAA receptor densities across cortical brain regions, which presumably contribute to cortical reorganization and the recovery of sensory-evoked activity (Garraghty et al., 2006; He et al., 2006; Mowery et al., 2013). In the auditory system, homeostatic mechanisms may support rebalanced excitation and inhibition in the IC and ACtx after developmental or adult hearing loss (Kotak et al., 2005; Yang et al., 2011; Sturm et al., 2017; Teichert et al., 2017) and altered distributions of AMPA and GABAA receptors have been reported to accompany cochlear trauma (Suneja et al., 2000; Holt et al., 2005; Dong et al., 2010a, 2010b; Browne et al., 2012).
The findings reported here identified two transcriptional changes that may underlie enhanced central gain following sudden hearing loss, but are by no means a complete description of the full set of changes. Acute hearing loss in adulthood shifts the mRNA and protein expression of multiple AMPA and GABAA receptor subunits, as well as other receptor types (Suneja et al., 1998, 2000; Milbrandt et al., 2000; Holt et al., 2005; Argence et al., 2006; Dong et al., 2010a; Smith et al., 2014) in addition to changes in voltage-gated channels that regulate intrinsic excitability (Yang et al., 2012; Li et al., 2015). Central gain-related changes in postsynaptic receptor densities are likely to vary by neuronal type, such that activity levels across some neural types are stable or suppressed, while others may become increasingly excitable (Takesian et al., 2013; Anderson et al., 2017; Sturm et al., 2017). Here, we used VGLUT and VGAT mRNA to coarsely group neurons into excitatory and inhibitory sub-classes (Figure 8), though we did not observe differences between these genetic categories in the STUD condition. A deeper analysis of cell-type specific shifts in Gria2 and Gabra1 expression, alongside the expression patterns of other subunits or receptor types, may elucidate the intracellular mechanisms that drive neuronal hyperactivity after peripheral deafferentation. A more comprehensive understanding of such mechanisms can potentially identify therapeutic targets for debilitating perceptual disorders such as tinnitus, hyperacusis, phantom limb pain, or visual release hallucinations.
Highlights.
AMPA and GABAA receptor mRNA levels were quantified in the adult mouse midbrain and cortex after unilateral hearing loss.
Thirty days after contralateral deprivation, individual neurons exhibited elevated AMPAR but reduced GABAAR mRNA.
Synergistic long-term shifts in mRNA support compensatory plasticity and were more pronounced in cortex than midbrain.
Degree of E/I transcriptional changes could not be predicted from the degree of hearing loss.
After just 5 days of hearing loss, AMPAR and GABAAR mRNA were proportionately reduced, causing no net shift in E/I.
Acknowledgements:
This work was supported by grants and fellowships from the National Institute of Deafness and Other Communication Disorders (DC009836 (DP), DC015388 (TH) and DC015710 (PB) as well as a research grant from Autifony Therapeutics (DP) and other financial support from the Lauer Tinnitus Research Center (DP). TAH developed initial RNAscope protocols. All authors contributed to experimental design. PB collected and analyzed the data. PB and DP wrote the manuscript. We thank J. L’Heureux and B. Robert for their help developing Matlab scripts for mRNA quantification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CT, Kumar M, Xiong S, Tzounopoulos T (2017) Cell-specific gain modulation by synaptically released zinc in cortical circuits of audition. eLife 6:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argence M, Saez I, Sassu R, Vassias I, Vidal PP, de Waele C (2006) Modulation of inhibitory and excitatory synaptic transmission in rat inferior colliculus after unilateral cochleectomy: An in situ and immunofluorescence study. Neuroscience 141:1193–1207. [DOI] [PubMed] [Google Scholar]
- Asokan MM, Williamson RS, Hancock KE, Polley DB (2018) Sensory overamplification in layer 5 auditory corticofugal projection neurons following cochlear nerve synaptic damage. Nat Commun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CJ, Morley JW, Parsons CH (2012) Tracking the expression of excitatory and inhibitory neurotransmission-related proteins and neuroplasticity markers after noise induced hearing loss Gilestro GF, ed. PLoS One 7:e33272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buran BN, Strenzke N, Neef A, Gundelfinger ED, Moser T, Liberman MC (2010) Onset coding Is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. J Neurosci 30:7587–7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Zhou X, Guo F, Xu J, Zhang J, Sun X (2010) Maintenance of enriched environment-induced changes of auditory spatial sensitivity and expression of GABAA, NMDA, and AMPA receptor subunits in rat auditory cortex. Neurobiol Learn Mem 94:452–460. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Eybalin M (1999) Glutamate receptor phenotypes in the auditory brainstem and mid-brain of the developing rat. Eur J Neurosci 11:51–74. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Ling LL (2013) Age-related GABAA receptor changes in rat auditory cortex. Neurobiol Aging 34:1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH (1995) Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol 30:349–360. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB (2016a) Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Salazar JJ, Polley DB (2016b) Persistent thalamic sound processing despite profound cochlear denervation. Front Neural Circuits 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Mulders WHAM, Rodger J, Robertson D (2009) Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neuroscience 159:1164–1174. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WHAM, Rodger J, Woo S, Robertson D (2010a) Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci 31:1616–1628. [DOI] [PubMed] [Google Scholar]
- Dong S, Rodger J, Mulders WHAM, Robertson D (2010b) Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res 1342:24–32. [DOI] [PubMed] [Google Scholar]
- Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG (2009) Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci 29:6479–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith G, Waschek J, Armstrong B, Edmond J, Lopez I, Liu W, Kurtz I (2006) Murine auditory brainstem evoked response: Putative two-channel differentiation of peripheral and central neural pathways. J Neurosci Methods 153:214–220. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Arnold LL, Wellman CL, Mowery TM (2006) Receptor autoradiographic correlates of deafferentation-induced reorganization in adult primate somatosensory cortex. J Comp Neurol 497:636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez a Khan ZU, Morris SJ De Blas a L (1994) Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J Neurosci 14:7469–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett T a., Clause AR, Takahata T, Hackett NJ, Polley DB (2015) Differential maturation of vesicular glutamate and GABA transporter expression in the mouse auditory forebrain during the first weeks of hearing. Brain Struct Funct 221:2619–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Rinaldi Barkat T, O’Brien BMJ, Hensch TK, Polley DB (2011) Linking topography to tonotopy in the mouse auditory thalamocortical circuit. J Neurosci 31:2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H-Y, Hodos W, Quinlan EM (2006) Visual Deprivation Reactivates Rapid Ocular Dominance Plasticity in Adult Visual Cortex. J Neurosci 26:2951–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA (2005) Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem 93:1069–1086. [DOI] [PubMed] [Google Scholar]
- Humanes-Valera D, Foffani G, Alonso-Calviño E, Fernández-López E, Aguilar J (2017) Dual Cortical Plasticity After Spinal Cord Injury. Cereb Cortex 27:2926–2940. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG (2008) Rapid Synaptic Scaling Induced by Changes in Postsynaptic Firing. Neuron 57:819–826. [DOI] [PubMed] [Google Scholar]
- Jaepel J, Hübener M, Bonhoeffer T, Rose T (2017) Lateral geniculate neurons projecting to primary visual cortex show ocular dominance plasticity in adult mice. Nat Neurosci 20:1708–1714. [DOI] [PubMed] [Google Scholar]
- Jiang C, Luo B, Manohar S, Chen G-D, Salvi R (2017) Plastic changes along auditory pathway during salicylate-induced ototoxicity: Hyperactivity and CF shifts. Hear Res 347:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Pons TP (1998) Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science 282:1121–1125. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Krubitzer LA, Chino YM, Langston AL, Polley EH, Blair N (1990) Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 248:229–231. [DOI] [PubMed] [Google Scholar]
- Kamke MR, Brown M, Irvine DRF (2003) Plasticity in the tonotopic organization of the medial geniculate body in adult cats following restricted unilateral cochlear lesions. J Comp Neurol 459:355–367. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MCW, Turrigiano GG (2002) Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci Neur 22:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH (2005) Hearing loss raises excitability in the auditory cortex. J Neurosci 25:3908–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH (1998) A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci 18:4646–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambo ME, Turrigiano GG (2013) Synaptic and Intrinsic Homeostatic Mechanisms Cooperate to Increase L2/3 Pyramidal Neuron Excitability during a Late Phase of Critical Period Plasticity. J Neurosci 33:8810–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA (2005) Ouabain induces apoptotic cell death in Type I spiral ganglion neurons, but not Type II neurons. J Assoc Res Otolaryngol 6:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kalappa BI, Tzounopoulos T (2015) Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. eLife 4:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D (2013) Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC (2007) Cellular/Molecular NMDA Receptor Activation Potentiates Inhibitory Transmission through GABA Receptor-Associated Protein- Dependent Exocytosis of GABA A Receptors. J Neurosci 27:14326–14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Guinan JJ, Knudson IM, Kiang NYS (1996) Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hear Res 93:28–51. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas J, Wall J, Nelson RJ, Sur M, Felleman D, Nelson RJ, Sur M, Felleman D (1983) Topographic reorganization of somatosensory cortical areas 3B and 1 in adult monkeys following restricted deafferentation. Neuroscience 8:33–55. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Caspary DM (1994) Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol Aging 15:699–703. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM (2000) GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res 147:251–260. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM (1997) Alterations of GABA A receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol 379:455–465. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Walls SM, Garraghty PE (2013) AMPA and GABAA/B receptor subunit expression in the cortex of adult squirrel monkeys during peripheral nerve regeneration. Brain Res 1520:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL (1998) Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21:1067–1078. [DOI] [PubMed] [Google Scholar]
- Petrus E, Rodriguez G, Patterson R, Connor B, Kanold PO, Lee H-K (2015) Vision loss shifts the balance of feedforward and intracortical circuits in opposite directions in mouse primary auditory and visual cortices. J Neurosci 35:8790–8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Salvi R, Ding D, Burkard R (2000) Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res 139:153–171. [DOI] [PubMed] [Google Scholar]
- Raza A, Milbrandt JC, Arneric SP, Caspary DM (1994) Age-related changes in brainstem auditory neurotransmitters: Measures of GABA and acetylcholine function. Hear Res 77:221–230. [DOI] [PubMed] [Google Scholar]
- Resnik J, Polley DB (2017) Fast-spiking GABA circuit dynamics in the auditory cortex predict recovery of sensory processing following peripheral nerve damage. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Irvine DRF (1989) Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol 282:456–471. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Kotak VC (2011) Developmental plasticity of auditory cortical inhibitory synapses. Hear Res 279:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC (1953) Hearing losses following partical section of the cochlear nerve. Laryngoscope 63:441–465. [DOI] [PubMed] [Google Scholar]
- Shim HJ, Lee LH, Huh Y, Lee SY, Yeo SG (2012) Age-related changes in the expression of NMDA, serotonin, and GAD in the central auditory system of the rat. Acta Otolaryngol 132:44–50. [DOI] [PubMed] [Google Scholar]
- Smith AR, Kwon JH, Navarro M, Hurley LM (2014) Acoustic trauma triggers upregulation of serotonin receptor genes. Hear Res 315:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm JJ, Zhang-Hooks Y-X, Roos H, Nguyen T, Kandler K (2017) Noise trauma induced behavioral gap detection deficits correlate with reorganization of excitatory and inhibitory local circuits in the inferior colliculus and are prevented by acoustic enrichment. J Neurosci 37:0602–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Mercado E, Wang P, Shan X, Lee T-C, Salvi RJ (2005) Changes in NMDA receptor expression in auditory cortex after learning. Neurosci Lett 374:63–68. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ (1998) Glycine Receptors in Adult Guinea Pig Brain Stem Auditory Nuclei: Regulation after Unilateral Cochlear Ablation. Exp Neurol 154:473–488. [DOI] [PubMed] [Google Scholar]
- Suneja SKK, Potashner SJJ, Benson CGG (2000) AMPA receptor binding in adult guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Exp Neurol 165:355–369. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sharma N, Sanes DH (2013) Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes. J Neurophysiol 110:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert M, Liebmann L, Hübner CA, Bolz J (2017) Homeostatic plasticity and synaptic scaling in the adult mouse auditory cortex. Sci Rep 7:17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G (2011) Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci 34:89–103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB (1998) Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391:892–896. [DOI] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y (2012) RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagnostics 14:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ (2002) Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res 168:238–249. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ruan Q, Wang D (2005) Different effects of intracochlear sensory and neuronal injury stimulation on expression of synaptic N-methyl-D-aspartate receptors in the auditory cortex of rats in vivo. Acta Otolaryngol 125:1145–1151. [DOI] [PubMed] [Google Scholar]
- Wekselblatt JB, Flister ED, Piscopo DM, Niell CM (2016) Large-scale imaging of cortical dynamics during sensory perception and behavior. J Neurophysiol 115:2852–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG (2005) Postsynaptic Expression of Homeostatic Plasticity at Neocortical Synapses. J Neurosci 25:2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Su W, Bao S (2012) Long-term, but not transient, threshold shifts alter the morphology and increase the excitability of cortical pyramidal neurons. J Neurophysiol 108:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho S-JS-J, Bao S (2011) Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci 108:14974–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C (2006) Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res 1099:73–81. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shi F, Yin Y, Tong M, Lang H, Polley DB, Liberman MC, Edge ASB (2013) Ouabain-induced cochlear nerve degeneration: synaptic loss and plasticity in a mouse model of auditory neuropathy. J Assoc Res Otolaryngol 15:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG (2005) Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol 93:3050–3063. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cudmore RH, Lin D-T, Linden DJ, Huganir RL (2015) Visualization of NMDA receptor–dependent AMPA receptor synaptic plasticity in vivo. Nat Neurosci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]