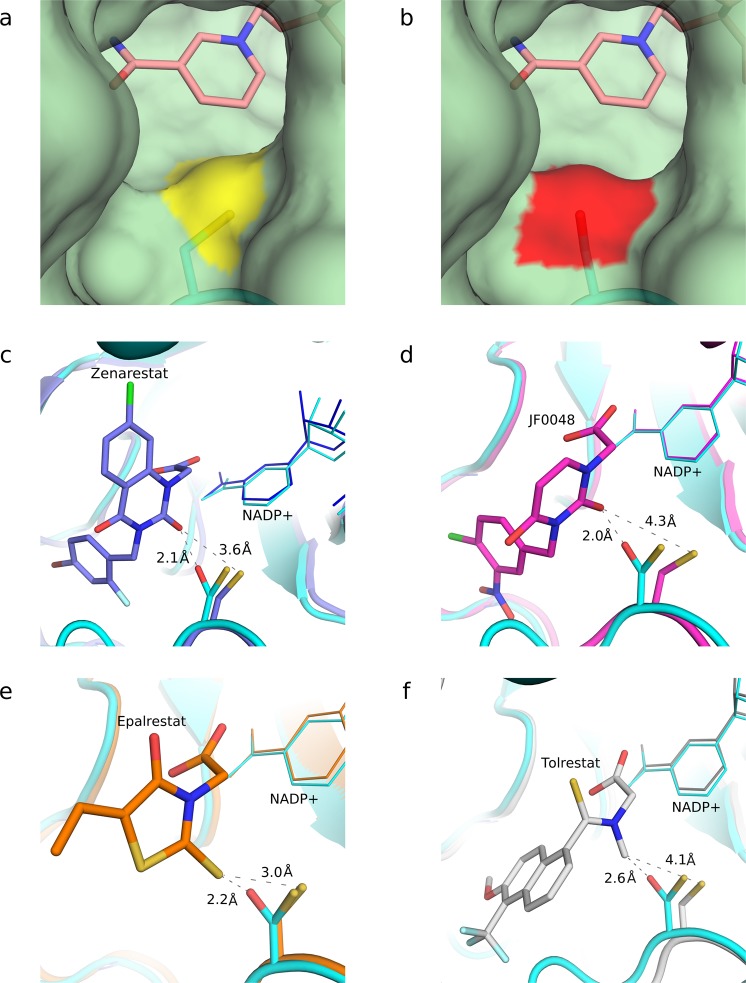
Figure 5.
Changes on the topology of the hAR pocket entry channel and steric collisions of activated hAR with inhibitors. The conversion of Cys298 into Ser298 modifies the entrance of the substrate-binding pocket. (a) Entrance of the binding pocket of the native form of hAR. The sulfur atom of the Cys298 residue is marked in yellow. (b) Entrance of the binding pocket of the irradiated structure, which shows the Oγ-Ser298 atom, marked in red. In both cases, the nicotinamide ring of the cofactor is shown in pink color. (c–f) Interaction of the studied inhibitors with Cys298 and Ser298. The structure of the D20 dataset (in cyan) is superimposed with the crystallographic structures of hAR including the inhibitors Zenarestat (c, purple, PDB 1IEI), JF0048 (d, magenta, PDB 4XZH), Epalrestat (e, orange, PDB 4JIR) and Tolrestat (f, gray, PDB 1AH3). In all cases the inhibitors, designed against the native form, show severe steric collisions with Oγ-Ser298 but not with Sγ-Cys298. Sulfur atoms are displayed in yellow whereas oxygen atoms are displayed in red.