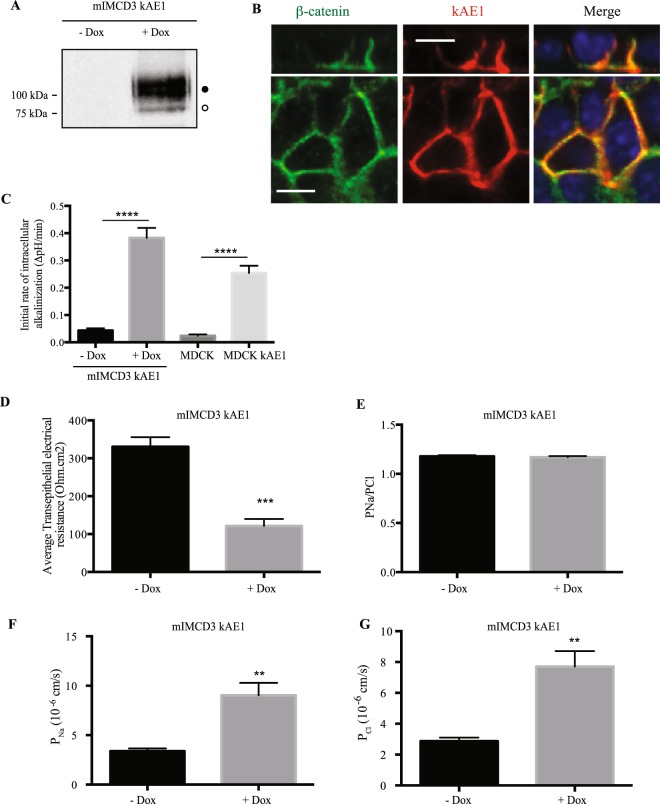
Figure 1.
Expression of kAE1 in mIMCD3 cells decreases trans-epithelial electrical resistance (TEER) and increases transepithelial sodium and chloride ion fluxes. (A) mIMCD3 cells expressing kAE1 in an inducible manner were incubated with or without doxycycline for 24 hours, prior to lysis and analysis of protein abundance by immunoblot. Mouse anti-HA antibody was used to detect kAE1 protein. Black and white circles correspond to kAE1 carrying complex and high mannose oligosaccharides, respectively. (B) mIMCD3 cells expressing kAE1 were grown to full polarization on semi-permeable filters for 10 days and induced with doxycycline for 24 hours prior to immunostaining with either anti-β-catenin antibody (green) or anti-HA antibody (red). The nuclei were stained with DAPI (blue). (C) 70% confluent mIMCD3 cells expressing kAE1 were induced with doxycycline for 24 hours prior to loading the cells with BCECF-AM in the presence of NaCl. Upon switching the extracellular solution to one containing Na gluconate instead of NaCl, the initial rate of intracellular alkalinization was recorded for the first 60 seconds and plotted as a function of time (see methods for further details). Error bars correspond to means ± SEM, n = 5 at minimum, ****P < 0.0001 versus “mIMCD3 kAE1 – Dox” or “MDCK” condition using one-way ANOVA. (D) Ussing chambers measurements of TEER showing that kAE1 expression results in decreased TEER but unchanged PNa/PCl ratio. (E) However, both absolute permeabilities to sodium (F) and chloride (G) increased upon kAE1 expression. Error bars correspond to means ± SEM, n = 4 at minimum, **P < 0.01 and ***P < 0.001 versus “– Dox” condition using un-paired t-test.