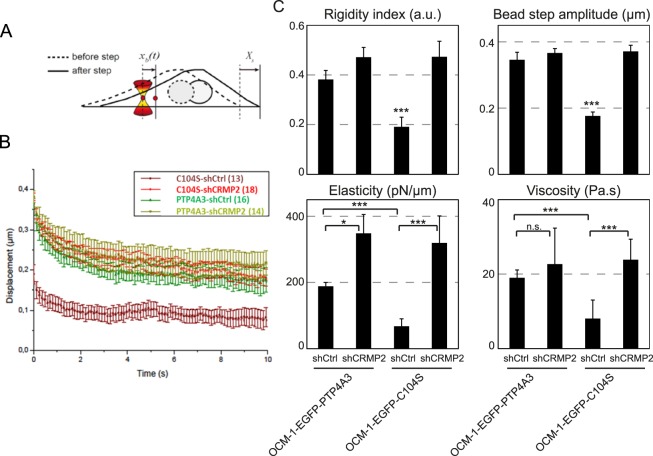
Figure 5.
CRMP2 affects the microrheological properties of the cells. (A) Sketch of the microrheology experiments. A 2-µm-diameter bead internalized in the cell is trapped with an optical tweezer. At time t = 0 s, the microscope stage is moved in a Xs = 0.5-µm step displacement. After the initial rapid displacement of the bead from the trap center, the bead position xb(t) relaxes towards the center of the optical trap, which acts as a spring. Single particle tracking of the bead allows determination of the viscoelastic relaxation curves shown in B. (B) Average bead displacement curves showing viscoelastic relaxation of the bead towards the trap center following a 0.5-µm step displacement of the microscope stage for OCM-1-EGFP-PTP4A3 and OCM1-EGFP-C104S cells treated with shCtrl or shCRMP2. (C) Quantification of the relaxation curves using a phenomenological model-independent approach (upper graphs) yielding the rigidity index and the bead-step amplitude, and the Standard Linear Liquid (SLL) viscoelastic model (lower graphs) yielding the elasticity and viscosity of the cytoplasm. Data were obtained from N = 16 and 14 beads for the OCM-1-EGFP-PTP4A3 cells treated with shCtrl or shCRMP2, respectively, and from N = 13 and 18 beads for OCM1-EGFP-C104S cells treated with shCtrl or shCRMP2, respectively. Error bars represent the standard error. p-values were determined using Student’s t-test for unpaired samples (***p < 0.001, *p < 0.05).